Unique challenges for glioblastoma immunotherapy-discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting Report from the 2019 SNO Immuno-Oncology Think Tank
- PMID: 33367885
- PMCID: PMC7992879
- DOI: 10.1093/neuonc/noaa277
Unique challenges for glioblastoma immunotherapy-discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting Report from the 2019 SNO Immuno-Oncology Think Tank
Abstract
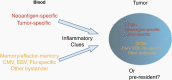
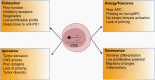
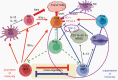
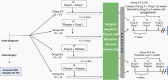
Cancer immunotherapy has made remarkable advances with over 50 separate Food and Drug Administration (FDA) approvals as first- or second-line indications since 2015. These include immune checkpoint blocking antibodies, chimeric antigen receptor-transduced T cells, and bispecific T-cell-engaging antibodies. While multiple cancer types now benefit from these immunotherapies, notable exceptions thus far include brain tumors, such as glioblastoma. As such, it seems critical to gain a better understanding of unique mechanistic challenges underlying the resistance of malignant gliomas to immunotherapy, as well as to acquire insights into the development of future strategies. An Immuno-Oncology Think Tank Meeting was held during the 2019 Annual Society for Neuro-Oncology Scientific Conference. Discussants in the fields of neuro-oncology, neurosurgery, neuro-imaging, medical oncology, and cancer immunology participated in the meeting. Sessions focused on topics such as the tumor microenvironment, myeloid cells, T-cell dysfunction, cellular engineering, and translational aspects that are critical and unique challenges inherent with primary brain tumors. In this review, we summarize the discussions and the key messages from the meeting, which may potentially serve as a basis for advancing the field of immune neuro-oncology in a collaborative manner.
Keywords: clinical trial; conference report; glioblastoma; immunosuppression; immunotherapy.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Weller M, Butowski N, Tran DD, et al. ; ACT IV Trial Investigators . Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. - PubMed
-
- Tocagen Reports Results of Toca 5 Phase 3 Trial in Recurrent Brain Cancer: Tocagen; 2019. https://www.biospace.com/article/releases/tocagen-reports-results-of-toc...

