Intestinal Antibody Responses to 2 Novel Live Attenuated Type 2 Oral Poliovirus Vaccines in Healthy Adults in Belgium
- PMID: 33367918
- PMCID: PMC9400418
- DOI: 10.1093/infdis/jiaa783
Intestinal Antibody Responses to 2 Novel Live Attenuated Type 2 Oral Poliovirus Vaccines in Healthy Adults in Belgium
Abstract
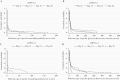
In a blinded phase 1 trial (EudraCT 2017-0000908-21; NCT03430349) in Belgium, healthy adults (aged 18-50 years) previously immunized exclusively with inactivated poliovirus vaccine were administered a single dose of 1 of 2 novel type 2 oral poliovirus vaccines (nOPV2-c1: S2/cre5/S15domV/rec1/hifi3 (n = 15); nOPV2-c2: S2/S15domV/CpG40 (n = 15)) and isolated for 28 days in a purpose-built containment facility. Using stool samples collected near days 0, 14, 21, and 28, we evaluated intestinal neutralization and immunoglobulin A responses to the nOPV2s and found that nOPV2-c1 and nOPV2-c2 induced detectable poliovirus type 2-specific intestinal neutralizing responses in 40.0% and 46.7% of participants, respectively.
Keywords: eradication; intestinal antibodies; live attenuated vaccine; mucosal immunity; poliovirus.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Brickley EB, Wieland-Alter W, Connor RI, et al. . Intestinal immunity to poliovirus following sequential trivalent inactivated polio vaccine/bivalent oral polio vaccine and trivalent inactivated polio vaccine-only immunization schedules: analysis of an open-label, randomized, controlled trial in Chilean infants. Clin Infect Dis 2018; 67:42–50. - PMC - PubMed
-
- Macklin GR, Grassly NC, Sutter RW, et al. . Vaccine schedules and the effect on humoral and intestinal immunity against poliovirus: a systematic review and network meta-analysis. Lancet Infect Dis 2019; 19:1121–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

