Population Pharmacodynamic Modeling Using the Sigmoid Emax Model: Influence of Inter-individual Variability on the Steepness of the Concentration-Effect Relationship. a Simulation Study
- PMID: 33367961
- PMCID: PMC7759489
- DOI: 10.1208/s12248-020-00549-7
Population Pharmacodynamic Modeling Using the Sigmoid Emax Model: Influence of Inter-individual Variability on the Steepness of the Concentration-Effect Relationship. a Simulation Study
Abstract
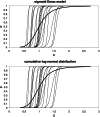
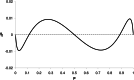
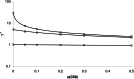
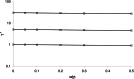
The relationship between the concentration of a drug and its pharmacological effect is often described by empirical mathematical models. We investigated the relationship between the steepness of the concentration-effect relationship and inter-individual variability (IIV) of the parameters of the sigmoid Emax model, using the similarity between the sigmoid Emax model and the cumulative log-normal distribution. In addition, it is investigated whether IIV in the model parameters can be estimated accurately by population modeling. Multiple data sets, consisting of 40 individuals with 4 binary observations in each individual, were simulated with varying values for the model parameters and their IIV. The data sets were analyzed using Excel Solver and NONMEM. An empirical equation (Eq. (11)) was derived describing the steepness of the population-predicted concentration-effect profile (γ*) as a function of γ and IIV in C50 and γ, and was validated for both binary and continuous data. The tested study design is not suited to estimate the IIV in C50 and γ with reasonable precision. Using a naive pooling procedure, the population estimates γ* are significantly lower than the value of γ used for simulation. The steepness of the population-predicted concentration-effect relationship (γ*) is less than that of the individuals (γ). Using γ*, the population-predicted drug effect represents the drug effect, for binary data the probability of drug effect, at a given concentration for an arbitrary individual.
Keywords: inter-individual variability; pharmacokinetic-pharmacodynamic modeling; sigmoid Emax model; simulation.
Figures
Similar articles
-
A technique for population pharmacodynamic analysis of concentration-binary response data.Anesthesiology. 1997 Apr;86(4):825-35. doi: 10.1097/00000542-199704000-00013. Anesthesiology. 1997. PMID: 9105227
-
Reliability of pharmacodynamic analysis by logistic regression: mixed-effects modeling.Anesthesiology. 2003 Dec;99(6):1255-62. doi: 10.1097/00000542-200312000-00005. Anesthesiology. 2003. PMID: 14639136
-
Population pharmacodynamics: strategies for concentration-and effect-controlled clinical trials.Ann Pharmacother. 1996 Jan;30(1):12-9. doi: 10.1177/106002809603000102. Ann Pharmacother. 1996. PMID: 8773159
-
Pharmacodynamic parameter estimation: population size versus number of samples.AAPS J. 2005 Oct 5;7(2):46. doi: 10.1208/aapsj070246. AAPS J. 2005. PMID: 16353905 Free PMC article. Review.
-
The efficiency concept in pharmacodynamics.Clin Pharmacokinet. 1999 May;36(5):375-89. doi: 10.2165/00003088-199936050-00005. Clin Pharmacokinet. 1999. PMID: 10384860 Review.
Cited by
-
Synergistic Effects of Korean Mistletoe and Apple Peel Extracts on Muscle Strength and Endurance.Nutrients. 2024 Sep 26;16(19):3255. doi: 10.3390/nu16193255. Nutrients. 2024. PMID: 39408221 Free PMC article.
-
Exposure-response analysis using time-to-event data for bevacizumab biosimilar SB8 and the reference bevacizumab.Front Pharmacol. 2024 Jan 16;14:1288308. doi: 10.3389/fphar.2023.1288308. eCollection 2023. Front Pharmacol. 2024. PMID: 38293674 Free PMC article.
References
-
- Greco WR, Park HS, Rustum YM. Application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinum and 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1990;50:5318–5327. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

