Effect of Preoperative Infusion of Levosimendan on Biomarkers of Myocardial Injury and Haemodynamics After Paediatric Cardiac Surgery: A Randomised Controlled Trial
- PMID: 33367965
- PMCID: PMC7937581
- DOI: 10.1007/s40268-020-00332-1
Effect of Preoperative Infusion of Levosimendan on Biomarkers of Myocardial Injury and Haemodynamics After Paediatric Cardiac Surgery: A Randomised Controlled Trial
Abstract
Objective: The aim was to test the hypothesis that preoperative infusion of levosimendan would decrease patients' cardiac biomarker profiles during the immediate postoperative stage (troponin I and B-type natriuretic peptide levels) more efficiently than placebo after cardiopulmonary bypass.
Methods: In a randomised, placebo-controlled, double-blinded study, 30 paediatric patients were scheduled for congenital heart disease surgery. 15 patients (50%) received prophylactic levosimendan and 15 patients (50%) received placebo from 12 h before cardiopulmonary bypass to 24 h after surgery.
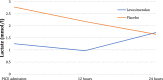
Results: Troponin I levels were higher in the placebo group at 0, 12, and 24 h after cardiopulmonary bypass, although the mean differences between the study groups and the 95% confidence intervals (CIs) for troponin I levels did not present statistically significant differences at any of the three time points considered (mean differences [95% CIs] - 3.32 pg/ml [- 19.34 to 12.70], - 2.42 pg/ml [- 19.78 to 13.95], and - 79.94 pg/ml [- 266.99 to 16.39] at 0, 12, and 24 h, respectively). A similar lack of statistically significant difference was observed for B-type natriuretic peptide (mean differences [95% CIs] 36.86 pg/dl [- 134.16 to 225.64], - 350.79 pg/dl [- 1459.67 to 557.45], and - 310.35 pg/dl [- 1505.76 to 509.82]). Lactic acid levels were significantly lower with levosimendan; the mean differences between the study groups and the 95% CIs for lactate levels present statistically significant differences at 0 h (- 1.52 mmol/l [- 3.19 to - 0.25]) and 12 h (- 1.20 mmol/l [- 2.53 to - 0.10]) after cardiopulmonary bypass. Oxygen delivery (DO2) was significantly higher at 12 h and 24 h after surgery (mean difference [95% CI] 627.70 ml/min/m2 [122.34-1162.67] and 832.35 ml/min/m2 [58.15 to 1651.38], respectively).
Conclusions: Levosimendan does not significantly improve patients' postoperative troponin I and B-type natriuretic peptide profiles during the immediate postoperative stage in comparison with placebo, although both were numerically higher with placebo. Levosimendan, however, significantly reduced lactic acid levels and improved patients' DO2 profiles. These results highlight the importance of this new drug and its possible benefit with regard to myocardial injury; however, evaluation in larger, adequately powered trials is needed to determine the efficacy of levosimendan. Trial registry number: EudraCT 2012-005310-19.
Conflict of interest statement
The authors, AA-M, JMG-L, FP, ME-M, AF-M, CF-G, and EO-H, declare that there is no conflict of interest.
Figures



References
-
- Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.CIR.0000051365.81920.28. - DOI - PubMed
-
- Pérez Vela JL, Martín Benítez JC, Carrasco González M, de la Cal López MA, Hinojosa Pérez R, Sagredo Meneses V, et al. Clinical practice guide for the management of low cardiac output syndrome in the postoperative period of heart surgery. Med Intensiva. 2012;36(4):e1–e44. doi: 10.1016/j.medin.2012.02.007. - DOI - PubMed

