Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia
- PMID: 33368143
- PMCID: PMC8728637
- DOI: 10.1002/14651858.CD012867.pub2
Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia
Update in
-
Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia.Cochrane Database Syst Rev. 2022 Mar 29;3(3):CD012867. doi: 10.1002/14651858.CD012867.pub3. Cochrane Database Syst Rev. 2022. PMID: 35349161 Free PMC article.
Abstract
Background: A variety of minimally invasive surgical approaches are available as an alternative to transurethral resection of the prostate (TURP) for management of lower urinary tract symptoms (LUTS) in men with benign prostatic hyperplasia (BPH). Prostatic arterial embolization (PAE) is a relatively new, minimally invasive treatment approach.
Objectives: To assess the effects of PAE compared to other procedures for treatment of LUTS in men with BPH.
Search methods: We performed a comprehensive search using multiple databases (The Cochrane Library, MEDLINE, Embase, LILACS, Scopus, Web of Science, and Google Scholar), trials registries, other sources of grey literature, and conference proceedings with no restrictions on language of publication or publication status, up until 25 September 2020.
Selection criteria: We included parallel-group randomized controlled trials (RCTs), as well as non-randomized studies (NRS, limited to prospective cohort studies with concurrent comparison groups) enrolling men over the age of 40 with LUTS attributed to BPH undergoing PAE versus TURP or other surgical interventions. DATA COLLECTION AND ANALYSIS: Two review authors independently classified studies for inclusion or exclusion and abstracted data from the included studies. We performed statistical analyses by using a random-effects model and interpreted them according to the Cochrane Handbook for Systematic Reviews of Interventions. We used GRADE guidance to rate the certainty of evidence of RCTs and NRSs. MAIN RESULTS: We found data to inform two comparisons: PAE versus TURP (six RCTs and two NRSs), and PAE versus sham (one RCT). Mean age, IPSS, and prostate volume of participants were 66 years, 22.8, and 72.8 mL, respectively. This abstract focuses on the comparison of PAE versus TURP as the primary topic of interest. PAE versus TURP We included six RCTs and two NRSs with short-term (up to 12 months) follow-up and one RCT with long-term follow-up (13 to 24 months). Short-term follow-up: based on RCT evidence, there may be little to no difference in urologic symptom score improvement (mean difference [MD] 1.55, 95% confidence interval [CI] -0.40 to 3.50; 369 participants; 6 RCTs; I² = 75%; low-certainty evidence) measured by the International Prostatic Symptom Score (IPSS) on a scale from 0 to 35, with higher scores indicating worse symptoms. There may be little to no difference in quality of life (MD 0.16, 95% CI -0.37 to 0.68; 309 participants; 5 RCTs; I² = 56%; low-certainty evidence) as measured by the IPSS quality of life question on a scale from 0 to 6, with higher scores indicating worse quality of life between PAE and TURP, respectively. While we are very uncertain about the effects of PAE on major adverse events (risk ratio [RR] 0.71, 95% CI 0.16 to 3.10; 250 participants; 4 RCTs; I² = 26%; very low-certainty evidence), PAE may increase re-treatments (RR 3.64, 95% CI 1.02 to 12.98; 204 participants; 3 RCTs; I² = 0%; low-certainty evidence). Based on 18 re-treatments per 1000 men in the TURP group, this corresponds to 47 more (0 more to 214 more) per 1000 men undergoing PAE. We are very uncertain about the effects on erectile function (MD -0.03, 95% CI -6.35 to 6.29; 129 participants; 2 RCTs; I² = 78%; very low-certainty evidence) measured by the International Index of Erectile Function at 5 on a scale from 1 to 25, with higher scores indicating better function. NRS evidence when available yielded similar results. Based on evidence from NRS, PAE may reduce the occurrence of ejaculatory disorders (RR 0.51, 95% CI 0.35 to 0.73; 260 participants; 1 NRS; low-certainty evidence). Longer-term follow-up: based on RCT evidence, we are very uncertain about the effects of PAE on urologic symptom scores (MD 0.30, 95% CI -3.17 to 3.77; 95 participants; very low-certainty evidence) compared to TURP. Quality of life may be similar (MD 0.20, 95% CI -0.49 to 0.89; 95 participants; low-certainty evidence). We are also very uncertain about major adverse events (RR 1.96, 95% CI 0.63 to 6.13; 107 participants; very low-certainty evidence). We did not find evidence on erectile function and ejaculatory disorders. Based on evidence from NRS, PAE may increase re-treatment rates (RR 1.51, 95% CI 0.43 to 5.29; 305 participants; low-certainty evidence); based on 56 re-treatments per 1000 men in the TURP group. this corresponds to 143 more (25 more to 430 more) per 1000 men in the PAE group. AUTHORS' CONCLUSIONS: Compared to TURP up to 12 months (short-term follow-up), PAE may provide similar improvement in urologic symptom scores and quality of life. While we are very uncertain about major adverse events, PAE may increase re-treatment rates. We are uncertain about erectile function, but PAE may reduce ejaculatory disorders. Longer term (follow-up of 13 to 24 months), we are very uncertain as to how both procedures compare with regard to urologic symptom scores, but quality of life appears to be similar. We are very uncertain about major adverse events but PAE may increase re-treatments. We did not find longer term evidence on erectile function and ejaculatory disorders. Certainty of evidence for the main outcomes of this review was low or very low, signalling that our confidence in the reported effect size is limited or very limited, and that this topic should be better informed by future research.
پیشینه: طیفی از رویکردهای جراحی کمتهاجمیتر بهعنوان گزینههای درمانی جایگزین رزکسیون پروستات از طریق مجاری ادراری (transurethral resection of the prostate; TURP) در مدیریت نشانههای دستگاه ادراری تحتانی (lower urinary tract symptoms; LUTS) در مردان مبتلا به هیپرپلازی خوشخیم پروستات (benign prostatic hyperplasia; BPH) پیشنهاد شدهاند. آمبولیزاسیون شریانی پروستات (prostatic arterial embolization; PAE) یک روش درمانی نسبتا جدید و کم تهاجمی است. اهداف: ارزیابی اثرات PAE در مقایسه با سایر روشهای درمان LUTS در مردان مبتلا به BPH. روشهای جستوجو: ما جستوجوی جامعی را در بانکهای اطلاعاتی مختلف (کتابخانه کاکرین، MEDLINE؛ Embase؛ LILACS؛ Scopus؛ Web of Science؛ و Google Scholar)، پایگاههای ثبت کارآزماییها، سایر منابع علمی منتشر نشده، و مجموعه مقالات کنفرانس منتشر شده تا 25 سپتامبر 2020، بدون اعمال محدودیت در زبان یا وضعیت انتشار، انجام دادیم. معیارهای انتخاب: ما کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs)، همچنین مطالعات غیر‐تصادفیسازی شده (NRS، محدود به مطالعات کوهورت آیندهنگر با گروههای مقایسه همزمان) را با حضور مردان بالای 40 سال مبتلا به LUTS وارد کردیم که نشانههایشان به BPH نسبت داده شد و تحت PAE در مقابل TURP یا سایر مداخلات جراحی قرار گفتند. گردآوری و تجزیهوتحلیل دادهها: دو نویسنده مرور بهطور مستقل از هم مطالعات را برای ورود و خروج طبقهبندی کرده و دادهها را از مطالعات وارد شده خلاصه کردند. ما تجزیهوتحلیل آماری را با استفاده از یک مدل اثرات تصادفی انجام دادیم و آنها را مطابق با کتابچه راهنمای کاکرین برای مرورهای سیستماتیک مداخلات تفسیر کردیم. برای ارزیابی قطعیت شواهد RCTها و NRSها از راهنمایی GRADE استفاده شد. نتایج اصلی: ما دادههایی را برای آگاهی از دو مقایسه یافتیم: PAE در مقابل TURP (شش RCT و دو NRS)، و PAE در مقابل روش ساختگی (یک RCT). میانگین سنی، IPSS، و حجم پروستات شرکتکنندگان، به ترتیب 66 سال، 22.8 و 72.8 میلیلیتر بود. این چکیده متمرکز است بر مقایسه PAE در مقابل TURP به عنوان موضوع اصلی مورد نظر. PAE در مقابل TURP ما شش RCT و دو NRS را با پیگیری کوتاهمدت (تا 12 ماه) و یک RCT را با پیگیری طولانیمدت (13 تا 24 ماه) وارد کردیم. پیگیری کوتاهمدت: بر اساس شواهد به دست آمده از RCT، ممکن است تفاوتی اندک یا عدم تفاوت در بهبود نمره نشانههای اورولوژیک وجود داشته باشد (تفاوت میانگین [MD]: 1.55؛ 95% فاصله اطمینان [CI]: 0.40‐ تا 3.50؛ 369 شرکتکننده؛ 6 RCT؛ I² = 75%؛ شواهد با قطعیت پائین) که با نمره بینالمللی نشانههای پروستات (International Prostatic Symptom Score; IPSS) در مقیاس 0 تا 35 اندازهگیری شد، و نمرات بالاتر نشانههای بدتر را نشان میدهد. در کیفیت زندگی نیز ممکن است تفاوتی اندک یا عدم تفاوت وجود داشته باشد (MD: 0.16؛ 95% CI؛ 0.37‐ تا 0.68؛ 309 شرکتکننده؛ 5 RCT؛ I² = 56%؛ شواهد با قطعیت پائین) که توسط سوال کیفیت زندگی IPSS در یک مقیاس 0 تا 6 اندازهگیری شد، و نمرات بالاتر نشانگر کیفیت زندگی بدتر به ترتیب بین PAE و TURP است. در حالی که ما در مورد تأثیر PAE بر عوارض جانبی عمده بسیار نامطمئن هستیم (خطر نسبی [RR]: 0.71؛ 95% CI؛ 0.16 تا 3.10؛ 250 شرکتکننده؛ 4 RCT؛ I² = 26%؛ شواهد با قطعیت بسیار پائین)، PAE ممکن است نیاز را به درمان مجدد افزایش دهد (RR: 3.64؛ 95% CI؛ 1.02 تا 12.98؛ 204 شرکتکننده؛ 3 RCT؛ I² = 0%؛ شواهد با قطعیت بسیار پائین). بر اساس 18 مورد درمان مجدد در هر 1000 مرد در گروه TURP، این عدد متناظر است با 47 مورد دیگر (0 مورد بیشتر تا 214 مورد بیشتر) در هر 1000 مرد تحت درمان با PAE. ما در مورد اثرات درمان بر عملکرد نعوظ بسیار نامطمئن هستیم (MD: ‐0.03؛ 95% CI؛ 6.35‐ تا 6.29؛ 129 شرکتکننده؛ 2 RCT؛ I² = 78%؛ شواهد با قطعیت بسیار پائین) که توسط شاخص بینالمللی عملکرد نعوظ در 5 روی مقیاس 1 تا 25 اندازهگیری شد، که نمرات بالاتر نشان دهنده عملکرد بهتر است. شواهد NRS در صورت وجود، نتایج مشابهی را به همراه داشت. براساس شواهد به دست آمده از NRS، روش PAE ممکن است بروز اختلالات انزال را کاهش دهد (RR: 0.51؛ 95% CI؛ 0.35 تا 0.73؛ 260 شرکتکننده؛ 1 NRS؛ شواهد با قطعیت پائین). پیگیری طولانیمدتتر: براساس شواهد RCT، ما در مورد تأثیر PAE بر نمرات نشانههای اورولوژیک، در مقایسه با TURP، بسیار نامطمئن هستیم (MD: 0.30؛ 95% CI؛ 3.17‐ تا 3.77؛ 95 شرکتکننده؛ شواهد با قطعیت بسیار پائین). کیفیت زندگی ممکن است مشابه باشد (MD: 0.20؛ 95% CI؛ 0.49‐ تا 0.89؛ 95 شرکتکننده؛ شواهد با قطعیت پائین). ما همچنین در مورد حوادث عمده جانبی بسیار نامطمئن هستیم (RR: 1.96؛ 95% CI؛ 0.63 تا 6.13؛ 107 شرکتکننده؛ شواهد با قطعیت بسیار پائین). ما شواهدی را در مورد عملکرد نعوظ و اختلالات انزال پیدا نکردیم. بر اساس شواهد به دست آمده از NRS، روش PAE ممکن است نرخ درمان مجدد را افزایش دهد (RR: 1.51؛ 95% CI؛ 0.43 تا 5.29؛ 305 شرکتکننده؛ شواهد با قطعیت پائین)؛ بر اساس 56 مورد نیاز به درمان مجدد در هر 1000 مرد در گروه TURP. این متناظر است با 143 نفر بیشتر (25 نفر بیشتر تا 430 نفر بیشتر) در هر 1000 مرد در گروه PAE. نتیجهگیریهای نویسندگان: در مقایسه با TURP تا 12 ماه (پیگیری کوتاهمدت)، PAE ممکن است میزان بهبودی مشابهی را در نمرات نشانههای اورولوژیک و کیفیت زندگی ایجاد کند. در حالی که در مورد عوارض جانبی عمده بسیار نامطمئن هستیم، PAE ممکن است نرخ درمان مجدد را افزایش دهد. ما در مورد عملکرد نعوظ اطمینان نداریم، اما PAE ممکن است اختلالات انزال را کاهش دهد. با پیگیری طولانیمدتتر (13 تا 24 ماه)، در مورد اینکه هر دو روش با توجه به نمرات نشانههای اورولوژیک چگونه مقایسه میشوند، بسیار نامطمئن هستیم، اما به نظر میرسد کیفیت زندگی مشابه باشد. در مورد عوارض جانبی عمده بسیار نامطمئن هستیم، اما PAE ممکن است نرخ درمان مجدد را افزایش دهد. شواهد طولانیمدتتری را در مورد عملکرد نعوظ و اختلالات انزال پیدا نکردیم. قطعیت شواهد برای پیامدهای اصلی این مرور، پائین یا بسیار پائین بود، این نشان دهنده آن است که اعتماد ما به اندازه تاثیرگذاری گزارش شده، محدود یا بسیار محدود است، و این موضوع باید در تحقیقات آینده بهتر بررسی شود.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JHJ: none.
MB: Boston Scientific (consultant for endourology and stone management), Auris Health (consultant for robotic surgery and endourology).
KAM: none.
SY: none.
JG: none.
MHK: none.
VN: none.
PD: none.
Figures

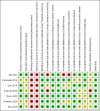
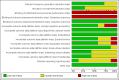
























References
References to studies included in this review
Abt 2021 {published data only}
-
- Abt D, Hechelhammer L, Müllhaupt G, Kessler T, Schmid HP, Engeler DS, et al.Prostatic artery embolization vs conventional TUR-P in the treatment of benign prostatic hyperplasia: first results of a prospective, randomized non-inferiority trial. European Urology Supplement 2016;15(3):e1080. [DOI: 10.1016/S1569-9056(16)61081-3] - DOI
-
- Abt D, Hechelhammer L, Müllhaupt G, Markart S, Güsewell S, Kessler TM, et al.Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ 2018;361:k2338. [DOI: 10.1136/bmj.k2338] - DOI - PMC - PubMed
-
- Abt D, Müllhaupt G, Hechelhammer L, Markart S, Güsewell S, Schmid HP, et al.Prostatic artery embolisation versus transurethral resection of the prostate for benign prostatic hyperplasia: 2-yr outcomes of a randomised, open-label, single-centre trial. European Urology 2021;80(1):34-42. [DOI: 10.1016/j.eururo.2021.02.008] - DOI - PubMed
-
- Abt D, Mordasini L, Hechelhammer L, Kessler TM, Schmid HP, Engeler DS.Prostatic artery embolization versus conventional TUR-P in the treatment of benign prostatic hyperplasia: protocol for a prospective randomized non-inferiority trial. BMC Urology 2014;14:94. [DOI: 10.1186/1471-2490-14-94] - DOI - PMC - PubMed
-
- Abt D, Mullhaupt G, Hechelhammer L, Markart S, Gusewell S, Schmid HP, et al.Prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: two-year outcomes of a randomised, open label, single-centre trial. European Urology 2021;79(Suppl 1):S83. - PubMed
Carnevale 2016 {published data only}
-
- Carnevale FC, Iscaife A, Yoshinaga EM, Moreira AM, Antunes AA, Srougi M.Transurethral resection of the prostate (TURP) versus original and perfected prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovascular and Interventional Radiology 2016;39(1):44-52. [DOI: 10.1007/s00270-015-1202-4] - DOI - PubMed
-
- Yoshinaga EM, Nakano E, Marchini GS, Galvao O, Baroni R, Carnevale FC, et al.A prospective and randomized trial comparing transurethral resection of the prostate (TURP) to prostate artery embolization (PAE) for treatment of bladder outlet obstruction due to benign prostatic hyperplasia (BPH). Journal of Urology 2014;191(4 Suppl):e793. [DOI: 10.1016/j.juro.2014.02.2168] - DOI
Gao 2014 {published data only}
Insausti 2020 {published data only}
-
- Giral Villalta PJ, Aguilar Guevara JF, Lopez Ubillos G, Lacarra Fernandez S, Zabalo San Juan A, Asiáin Urmeneta M, et al.Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: 12 month results of a clinical trial. European Urology, Supplements 2019;18(1):e1494-5. [DOI: 10.1016/S1569-9056(19)31075-9] - DOI
-
- Insausti I, Sáez de Ocáriz García A, Galbete A, Capdevila F, Solchaga S, Giral P, et al.Randomized comparison of prostatic arterial embolization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology 2020;31(6):882-90. [DOI: 10.1016/j.jvir.2019.12.810] - DOI - PubMed
-
- Napal Lecumberri S, Insausti Gorbea I, Sáez de Ocáriz García A, Solchaga Álvarez S, Cebrián Lostal JL, Monreal Beortegui R, et al.Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: protocol for a non-inferiority clinical trial. Research and Reports in Urology 2018;10:17-22. [DOI: 10.2147/RRU.S139086] - DOI - PMC - PubMed
-
- NCT01963312.Clinical trial to evaluate the efficacy and safety of the transarterial supraselective embolization of the prostate to treat the urinary symptoms. clinicaltrials.gov/ct2/show/NCT01963312 (first received 16 October 2013).
Pisco 2020 {published data only}
-
- NCT02074644.Clinical trial of prostatic arterial embolization versus a sham procedure to treat benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT02074644 (first received 28 February 2014).
Radwan 2020 {published data only}
Ray 2018 {published data only}
-
- Dasgupta R, Speakman M, Ray A, Powell J, Modi S, Carolan-Rees G, et al.Prostate artery embolisation versus TURP; a multicentric prospective comparison: the UK-ROPE study. Journal of Urology 2018;199(4 Suppl):e835. [DOI: 10.1016/j.juro.2018.02.2010] - DOI
-
- Modi S, Bryant TJ, Ray AF, Hacking N.UK-ROPE: preliminary findings. Cardiovascular and Interventional Radiology 2016;39(3):S152.
-
- NCT02434575.UK ROPE Register Study. clinicaltrials.gov/show/NCT02434575 (first received 5 May 2015).
-
- NCT02849522.ROPE registry project to determine the safety and efficacy of prostate artery embolisation (PAE) for lower urinary tract symptoms secondary to benign prostatic enlargement (LUTS BPE). clinicaltrials.gov/show/NCT02849522 (first received 29 July 2016).
-
- Ray AF, Powell J, Speakman MJ, Longford NT, DasGupta R, Bryant T, et al.Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU International 2018;122(2):270-82. [DOI: 10.1111/bju.14249] - DOI - PubMed
Soluyanov 2018 {published data only}
-
- Soluyanov MY, Shumkov OA, Smagin MA, Nimaev VV.First experience with prostate artery embolization for benign prostatic hyperplasia. Urologia 2018;4:33-7. - PubMed
Zhu 2018 {published data only}
-
- Zhu C, Lin W, Huang Z, Cai J.Prostate artery embolization and transurethral resection of prostate for benign prostatic hyperplasia: a prospective randomized controlled trial. Chinese Journal of Interventional Imaging and Therapy 2018;15(3):134-8. [DOI: 10.13929/j.1672-8475.201711043] - DOI
References to studies excluded from this review
Abt 2019 {published data only}
-
- Abt D, Mullhaupt G, Mordasini L, Gusewell S, Markart S, Zumstein V, et al.Outcome prediction of prostatic artery embolization: post hoc analysis of a randomized, open-label, non-inferiority trial. BJU International 2019;124(1):134-44. - PubMed
Bagla 2017 {published data only}
-
- Bagla S, Smirniotopoulos J, Orlando J, Piechowiak R.Cost analysis of prostate artery embolization (PAE) and transurethral resection of the prostate (TURP) in the treatment of benign prostatic hyperplasia. Cardiovascular and Interventional Radiology 2017;40(11):1694-7. [DOI: 10.1007/s00270-017-1700-7] - DOI - PubMed
-
- Bagla S, Vadlamudi V, Orlando J, Smirniotopoulos J.Cost analysis of prostate artery embolization (PAE) and transurethral resection of the prostate (TURP) in the treatment of benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology 2016;27(3):S56. [DOI: 10.1016/j.jvir.2015.12.154] - DOI - PubMed
Bilhim 2015 {published data only}
-
- Bilhim T, Bagla S, Sapoval M, Carnevale FC, Salem R, Golzarian J.Prostatic arterial embolization versus transurethral resection of the prostate for benign prostatic hyperplasia. Radiology 2015;276(1):310-1. - PubMed
Brown 2019 {published data only}
Mullhaupt 2019 {published data only}
-
- Mullhaupt G, Hechelhammer L, Engeler DS, Gusewell S, Betschart P, Zumstein V, et al.In-hospital cost analysis of prostatic artery embolization compared with transurethral resection of the prostate: post hoc analysis of a randomized controlled trial. BJU International 2019;123(6):1055-60. - PMC - PubMed
NCT01835860 {unpublished data only}
-
- NCT01835860.Prostatic artery embolization for benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT01835860 (first received 19 April 2013).
NCT02006303 {unpublished data only}
-
- NCT02006303.Prostatic artery embolization versus 532 nm green light PVP for catheterized patients. clinicaltrials.gov/ct2/show/NCT02006303 (first received 10 December 2013).
NCT02566551 {unpublished data only}
-
- NCT02566551.Prospective controlled randomized study of PAE vs TURP for BPH treatment. clinicaltrials.gov/ct2/show/NCT02566551 (first received 2 October 2015).
Pereira 2018 {published data only}
-
- NCT03043222.Innovative minimally invasive options in treatment of urinary problems related to prostate enlargement (BPH) in men. clinicaltrials.gov/show/NCT03043222 (first received 3 February 2017).
-
- Pereira K, Ford-Glanton S, Johar R, Xu P, Pham K, Gadani S, et al.Prostatic artery embolization (PAE) and prostatic urethral lift (PUL) procedures for symptomatic benign prostatic enlargement (BPH): a retrospective, single-center comparison of outcomes. Journal of Vascular and Interventional Radiology 2018;29(4 Suppl 1):S6. [DOI: 10.1016/j.jvir.2018.01.010] - DOI
Qiu 2017 {published data only}
-
- Qiu ZL, Zhang CC, Wang XS, Cheng K, Liang X, Wang DW, et al.Clinical evaluation of embolization of the superior vesical prostatic artery for treatment of benign prostatic hyperplasia: a single-center retrospective study. Wideochir Inne Tech Maloinwazyjne 2017;12(4):409-16. [DOI: 10.5114/wiitm.2017.72324] - DOI - PMC - PubMed
Russo 2015 {published data only}
-
- Russo GI, Kurbatov D, Sansalone S, Lepetukhin A, Dubsky S, Sitkin I, et al.Prostatic arterial embolization vs open prostatectomy: a 1-year matched-pair analysis of functional outcomes and morbidities. Urology 2015;86(2):343-8. - PubMed
-
- Russo GI, Kurbatov D, Sansalone S, Lepetukhin A, Dubsky S, Sitkin I, et al.Prostatic arterial embolization vs open prostatectomy: a matched-pair analysis of functional outcomes and morbidities after 1 year of follow-up. European Urology Supplement 2015;14(2):e570. - PubMed
Steurer 2018 {published data only}
-
- Steurer J.Benign prostatic hyperplasia: transurethral resection or embolization of prostate arteries? Praxis 2018;107(20):1115-6. - PubMed
Wu 2019 {published data only}
-
- Wu S, Cai S, Yi T, Cai W, Zhou Y, He J, et al.Interventional embolization vs transurethral resection for the treatment of benign prostatic hyperplasia in elderly patients: a comparison study. Journal of Interventional Radiology 2019;28(2):179-83.
References to studies awaiting assessment
Ng 2020 {published data only}
-
- Ng CM, Chung K, Cheng B, Chak H, Chan W, Ming S, et al.Comparison of prostatic artery embolisation with transurethral resection of the prostate for high-risk surgical candidates in obstructive uropathy or refractory retention: a prospective cohort study. International Journal of Urology 2020;27(Suppl 1):113.
References to ongoing studies
ACTRN12617001235392 {unpublished data only}
-
- ACTRN12617001235392.Prostate artery embolization for patients with lower urinary tract symptoms due to benign prostate hyperplasia. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373427 (first received 24 August 2017).
ChiCTR1800014818 {unpublished data only}
-
- ChiCTR1800014818.Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia. www.chictr.org.cn/com/25/showprojen.aspx?proj=25226 (first received 7 February 2018).
NCT01789840 {unpublished data only}
-
- NCT01789840.Prostate artery embolization with embosphere microspheres compared to TURP for benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT01789840 (first received 12 February 2013).
NCT04084938 {unpublished data only}
-
- NCT04084938.Artery embolization vs operation of benign prostate hyperplasia (NORTAPE). clinicaltrials.gov/ct2/show/NCT04084938 (first received 10 September 2019).
NCT04236687 {unpublished data only}
-
- NCT04236687.Prostate artery embolization compared to holmium laser enucleation of the prostate for benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT04236687 (firs received 22 January 2020).
NCT04807010 {unpublished data only}
-
- NCT04807010.PROARTE -PROstate ARTery to reduce the symptoms of benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT04807010 (first received 19 March 2021). [NCT04807010]
Additional references
Ahyai 2010
-
- Ahyai SA, Gilling P, Kaplan SA, Kuntz RM, Madersbacher S, Montorsi F, et al.Meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic enlargement. European Urology 2010;58(3):384-97. - PubMed
Barry 1995
-
- Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al.Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? Journal of Urology 1995;154(5):1770-4. - PubMed
Barry 1997
-
- Barry MJ, Fowler FJ Jr, Bin L, Pitts JC 3rd, Harris CJ, Mulley AG Jr.The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. Journal of Urology 1997;157(1):10-4; discussion 14-5. - PubMed
Bhatia 2018
-
- Bhatia S, Sinha VK, Harward S, Gomez C, Kava BR, Parekh DJ.Prostate artery embolization in patients with prostate volumes of 80 mL or more: a single-institution retrospective experience of 93 patients. Journal of Vascular and Interventional Radiology 2018;29(10):1392-8. [DOI: 10.1016/j.jvir.2018.05.012] - DOI - PubMed
Bhojani 2014
-
- Bhojani N, Gandaglia G, Sood A, Rai A, Pucheril D, Chang SL, et al.Morbidity and mortality after benign prostatic hyperplasia surgery: data from the American College of Surgeons national surgical quality improvement program. Journal of Endourology 2014;28(7):831-40. - PubMed
Brasure 2016
-
- Brasure M, MacDonald R, Dahm P, Olson CM, Nelson VA, Fink HA, et al.AHRQ comparative effectiveness reviews. In: Newer Medications for Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: a Review. Rockville (MD): Agency for Healthcare Research and Quality (US), 2016. - PubMed
Carnevale 2010
-
- Carnevale FC, Antunes AA, da Motta Leal Filho JM, Oliveira Cerri LM, Baroni RH, Marcelino AS, et al.Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovascular and Interventional Radiology 2010;33(2):355-61. - PMC - PubMed
Centers for Disease Control and Prevention 2003
-
- Centers for Disease Control and Prevention.Public health and aging: trends in aging – United States and worldwide. JAMA 2003;289(11):1371-3. - PubMed
Chapple 2017
Covidence 2017 [Computer program]
-
- Veritas Health Innovation Covidence.Version accessed 18 August 2017. Melbourne, Australia: Veritas Health Innovation, 2013. Available at www.covidence.org.
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG, editor(s).Chapter 9. Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
DeMeritt 2000
-
- DeMeritt JS, Elmasri FF, Esposito MP, Rosenberg GS.Relief of benign prostatic hyperplasia-related bladder outlet obstruction after transarterial polyvinyl alcohol prostate embolization. Journal of Vascular and Interventional Radiology 2000;11(6):767-70. - PubMed
Dindo 2004
Dunphy 2015
EAU 2021
-
- European Association of Urology.Management of non-neurogenic male LUTS. uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ (accessed 3 November 2021).
Egan 2016
-
- Egan KB.The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urologic Clinics of North America 2016;43(3):289-97. - PubMed
EndNote 2016 [Computer program]
-
- EndNote.Version 7.5. Clarivate Analytics, 2016.
Feinsten 2018
-
- Feinsten L, Matlaga B.Urologic diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office, 2018; NIH Publication No. 12-7865.
Feng 2017
-
- Feng S, Tian Y, Liu W, Li Z, Deng T, Li H, et al.Prostatic arterial embolization treating moderate-to-severe lower urinary tract symptoms related to benign prostate hyperplasia: a meta-analysis. Cardiovascular and Interventional Radiology 2017;40(1):22-32. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT.Version accessed 18 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2008
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al.GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. - PubMed
Guyatt 2011b
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG.Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA.Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Johnston 2010
Jung 2019
-
- Jung JH, Reddy B, McCutcheon KA, Borofsky M, Narayan V, Kim MH, et al.Prostatic urethral lift for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD012832. [DOI: 10.1002/14651858.CD012832.pub2] - DOI - PMC - PubMed
Kang 2020
-
- Kang TW, Jung JH, Hwang EC, Borofsky M, Kim MH, Dahm P.Convective radiofrequency water vapour thermal therapy for lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013251. [DOI: 10.1002/14651858.CD013251.pub2] - DOI - PMC - PubMed
Kuang 2017
-
- Kuang M, Vu A, Athreya S.A systematic review of prostatic artery embolization in the treatment of symptomatic benign prostatic hyperplasia. Cardiovascular and Interventional Radiology 2017;40(5):655-63. - PubMed
Lerner 2021a
-
- Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, et al.Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART I – initial work-up and medical management. Journal of Urology 2021;206(4):806-17. - PubMed
Lerner 2021b
-
- Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, et al.Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART II – surgical evaluation and treatment. Journal of Urology 2021;206(4):818-26. - PubMed
Liberati 2009
Malaeb 2012
Malling 2019
-
- Malling B, Røder MA, Brasso K, Forman J, Taudorf M, Lönn L.Prostate artery embolisation for benign prostatic hyperplasia: a systematic review and meta-analysis. European Radiology 2019;29:287-98. - PubMed
Martin 2014
-
- Martin S, Lange K, Haren MT, Taylor AW, Wittert G.Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. Journal of Urology 2014;191(1):130-7. - PubMed
Martins Pisco 2012
-
- Martins Pisco J, Pereira J, Rio Tinto H, Fernandes L, Bilhim T.How to perform prostatic arterial embolization. Techniques in Vascular and Interventional Radiology 2012;15(4):286-9. - PubMed
McVary 2018
-
- McVary KT, Roehrborn CG.Three-year outcomes of the prospective, randomized controlled Rezūm System study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology 2018;111:1-9. - PubMed
McWilliams 2019
-
- McWilliams JP, Bilhim TA, Carnevale FC, Bhatia S, Isaacson AJ, Bagla S, et al.Society of Interventional Radiology multisociety consensus position statement on prostatic artery embolization for treatment of lower urinary tract symptoms attributed to benign prostatic hyperplasia: from the Society of Interventional Radiology, the Cardiovascular and Interventional Radiological Society of Europe, Société Française de Radiologie, and the British Society of Interventional Radiology: endorsed by the Asia Pacific Society of Cardiovascular and Interventional Radiology, Canadian Association for Interventional Radiology, Chinese College of Interventionalists, Interventional Radiology Society of Australasia, Japanese Society of Interventional Radiology, and Korean Society of Interventional Radiology. Journal of Vascular and Interventional Radiology 2019;30(5):627-37. - PubMed
Mitchell 1976
-
- Mitchell ME, Waltman AC, Athanasoulis CA, Kerr WS Jr, Dretler SP.Control of massive prostatic bleeding with angiographic techniques. Journal of Urology 1976;115(6):692-5. - PubMed
Narayan 2017
NICE 2018
-
- National Institute for Health and Care Excellence.Prostate artery embolisation for lower urinary tract symptoms caused by benign prostatic hyperplasia. Interventional procedures guidance. www.nice.org.uk/guidance/ipg611 (accessed 4 December 2018).
Nickel 2015
-
- Nickel JC, Brock GB, Herschorn S, Dickson R, Henneges C, Viktrup L.Proportion of tadalafil-treated patients with clinically meaningful improvement in lower urinary tract symptoms associated with benign prostatic hyperplasia – integrated data from 1,499 study participants. BJU International 2015;115(5):815-21. - PubMed
Omar 2014
-
- Omar MI, Lam TB, Alexander CE, Graham J, Mamoulakis C, Imamura M, et al.Systematic review and meta-analysis of the clinical effectiveness of bipolar compared with monopolar transurethral resection of the prostate (TURP). BJU International 2014;113(1):24-35. - PubMed
Pariser 2015
-
- Pariser JJ, Pearce SM, Patel SG, Bales GT.National trends of simple prostatectomy for benign prostatic hyperplasia with an analysis of risk factors for adverse perioperative outcomes. Urology 2015;86(4):721-5. - PubMed
Parsons 2015
-
- Parsons JK, Rangarajan SS, Palazzi K, Chang D.A national, comparative analysis of perioperative outcomes of open and minimally invasive simple prostatectomy. Journal of Endourology 2015;29(8):919-24. - PubMed
Pisco 2016
-
- Pisco JM, Bilhim T, Pinheiro LC, Fernandes L, Pereira J, Costa NV, et al.Medium- and long-term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. Journal of Vascular and Interventional Radiology 2016;27(8):1115-22. - PubMed
Pyo 2017
-
- Pyo JS, Cho WJ.Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clinical Radiology 2017;72(1):16-22. - PubMed
Rees 2015
-
- Rees J.Patients not P values. BJU international 2015;115(5):678-9. - PubMed
Reich 2008
-
- Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al.Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. Journal of Urology 2008;180(1):246-9. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roehrborn 2008
-
- Roehrborn CG.Pathology of benign prostatic hyperplasia. International Journal of Impotence Research 2008;20 Suppl 3:S11-8. - PubMed
Roehrborn 2017
-
- Roehrborn CG, Barkin J, Gange SN, Shore ND, Giddens JL, Bolton DM, et al.Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Canadian Journal of Urology 2017;24(3):8802-13. - PubMed
Rosen 1997
-
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A, et al.The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822-30. - PubMed
Rosen 2007
-
- Rosen RC, Catania JA, Althof SE, Pollack LM, O'Leary M, Seftel AD, et al.Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007;69(5):805-9. - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH.Chapter 11. Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al.Chapter 12. Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2013
-
- Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al.Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013;4(1):49-62. - PubMed
Schünemann 2019
Shim 2017
-
- Shim SR, Kanhai KJ, Ko YM, Kim JH.Efficacy and safety of prostatic arterial embolization: systematic review with meta-analysis and meta-regression. Journal of Urology 2017;197(2):465-79. - PubMed
Spaliviero 2010
-
- Spaliviero M, Strom KH, Gu X, Araki M, Culkin DJ, Wong C.Does Greenlight HPS(™) laser photoselective vaporization prostatectomy affect sexual function? Journal of Endourology 2010;24(12):2051-7. - PubMed
Sterne 2016a
Sterne 2016b
-
- Sterne JA, Higgins JP, Elbers RG, Reeves BC, Development group for ROBINS-I.Risk of bias In non-randomized studies of interventions (ROBINS-I): detailed guidance, updated 12 October 2016. www.riskofbias.info (accessed 9 November 2018).
Sun 2008
-
- Sun F, Sanchez FM, Crisostomo V, Lima JR, Luis L, Garcia-Martinez V, et al.Benign prostatic hyperplasia: transcatheter arterial embolization as potential treatment – preliminary study in pigs. Radiology 2008;246(3):783-9. - PubMed
Taub 2006
-
- Taub DA, Wei JT.The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Current Urology Reports 2006;7(4):272-81. - PubMed
Wang 2015
WHO 2002
-
- World Health Organization.Proposed working definition of an older person in Africa for the MDS project. www.who.int/healthinfo/survey/ageingdefnolder/en (accessed 17 August 2017).
Xu 2020
Yoo 2012
Young 2017
-
- Young S, Golzarian J.Prostate arterial embolization is a viable option for treating symptoms of benign prostatic hyperplasia: pro. Journal of Urology 2017;198(1):9-11. - PubMed
Zlotta 1997
-
- Zlotta AR, Raviv G, Peny MO, Noel JC, Haot J, Schulman CC.Possible mechanisms of action of transurethral needle ablation of the prostate on benign prostatic hyperplasia symptoms: a neurohistochemical study. Journal of Urology 1997;157(3):894-9. - PubMed
Zumstein 2019
-
- Zumstein V, Betschart P, Vetterlein MW, Kluth LA, Hechelhammer L, Mordasini L, et al.Prostatic artery embolization versus standard surgical treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. European Urology Focus 2019;5(6):1091-100. - PubMed
References to other published versions of this review
Jung 2017
-
- Jung JH, Shin TY, McCutcheon KA, Borofsky M, Narayan V, Young S, et al.Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012867. [DOI: 10.1002/14651858.CD012867] - DOI - PMC - PubMed
Jung 2020
-
- Jung JH, McCutcheon KA, Borofsky M, Young S, Golzarian J, Reddy B, et al.Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD012867. [DOI: 10.1002/14651858.CD012867.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

