A predictive model for estimating the number of erythrocytapheresis or phlebotomy treatments for patients with naïve hereditary hemochromatosis
- PMID: 33368569
- PMCID: PMC8247321
- DOI: 10.1002/jca.21867
A predictive model for estimating the number of erythrocytapheresis or phlebotomy treatments for patients with naïve hereditary hemochromatosis
Abstract
Background and aims: Standard treatment for naïve hereditary hemochromatosis patients consists of phlebotomy or a personalized erythrocytapheresis. Erythrocytapheresis is more efficient, but infrequently used because of perceived costs and specialized equipment being needed. The main aim of our study was to develop a model that predicts the number of initial treatment procedures for both treatment methods. This information may help the clinician to select the optimal treatment modality for the individual patient.
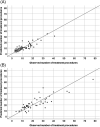
Methods: We analyzed retrospective data of 125 newly diagnosed patients (C282Y homozygous), treated either with phlebotomy (n = 54) or erythrocytapheresis (n = 71) until serum ferritin (SF) reached levels ≤100 μg/L. To estimate the required number of treatment procedures multiple linear regression analysis was used for each treatment method separately.
Results: The linear regression model with the best predictive quality (R2 = 0.74 and 0.73 for erythrocytapheresis and phlebotomy respectively) included initial SF, initial hemoglobin (Hb) level, age, and BMI, where initial SF was independently related to the total number of treatment procedures for both treatment methods. The prediction error expressed in RMSPE and RMSDR was lower for erythrocytapheresis than for phlebotomy (3.8 and 4.1 vs 7.0 and 8.0 respectively), CONCLUSIONS: Although the prediction error of the developed model was relatively large, the model may help the clinician to choose the most optimal treatment method for an individual patient. Generally erythrocytapheresis halves the number of treatment procedures for all patients, where the largest reduction (between 55% and 64%) is reached in patients with an initial Hb level ≥ 9 mmol/L (14.5 g/dL). ClinicalTrials.gov number NCT00202436.
Keywords: erythrocytapheresis; hereditary hemochromatosis; phlebotomy; prediction rule.
© 2020 The Authors. Journal of Clinical Apheresis published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to Journal of Clinical Apheresis.
Figures
References
-
- Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393‐408. - PubMed
-
- Ellervik C, Birgens H, Tybjaerg‐Hansen A, Nordestgaard BG. Hemochromatosis genotypes and risk of 31 disease endpoints: meta‐analyses including 66 000 cases and 226 000 controls. Hepatology. 2007;46:1071‐1080. - PubMed
-
- Adams PC, Barton JC. How I treat hemochromatosis. Blood. 2010;116:317‐325. - PubMed
-
- Rombout‐Sestrienkova E, van Kraaij MG, Koek GH. How we manage patients with hereditary hemochromatosis. Br J Haematol. 2016;175(5):759‐770. - PubMed
-
- Ong SY, Gurrin LC, Dolling L, et al. Reduction of body iron in HFE‐related haemochromatosis and moderate iron overload (Mi‐iron): a multicenter, participant‐blinded, randomized controlled trial. Lancet Haematol. 2017;4:e 607‐e 614. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

