Subject-specific segregation of functional territories based on deep phenotyping
- PMID: 33368868
- PMCID: PMC7856658
- DOI: 10.1002/hbm.25189
Subject-specific segregation of functional territories based on deep phenotyping
Abstract
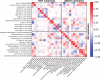
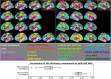
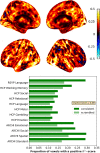
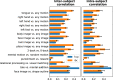
Functional magnetic resonance imaging (fMRI) has opened the possibility to investigate how brain activity is modulated by behavior. Most studies so far are bound to one single task, in which functional responses to a handful of contrasts are analyzed and reported as a group average brain map. Contrariwise, recent data-collection efforts have started to target a systematic spatial representation of multiple mental functions. In this paper, we leverage the Individual Brain Charting (IBC) dataset-a high-resolution task-fMRI dataset acquired in a fixed environment-in order to study the feasibility of individual mapping. First, we verify that the IBC brain maps reproduce those obtained from previous, large-scale datasets using the same tasks. Second, we confirm that the elementary spatial components, inferred across all tasks, are consistently mapped within and, to a lesser extent, across participants. Third, we demonstrate the relevance of the topographic information of the individual contrast maps, showing that contrasts from one task can be predicted by contrasts from other tasks. At last, we showcase the benefit of contrast accumulation for the fine functional characterization of brain regions within a prespecified network. To this end, we analyze the cognitive profile of functional territories pertaining to the language network and prove that these profiles generalize across participants.
Keywords: atlases; brain imaging; cognitive function; data set; functional magnetic resonance imaging.
© 2020 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Figures









References
-
- Abraham, A. , Dohmatob, E. , Thirion, B. , Samaras, D. , & Varoquaux, G. (2013). Extracting brain regions from rest fMRI with total‐variation constrained dictionary learning. In MICCAI—16th International Conference on Medical Image Computing and Computer Assisted Intervention ‐ 2013 Nagoya, Japan: Springer. 10.1007/978-3-642-40763-5.75 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

