Transmitted HIV-1 drug resistance in a large international cohort using next-generation sequencing: results from the Strategic Timing of Antiretroviral Treatment (START) study
- PMID: 33369017
- PMCID: PMC8049964
- DOI: 10.1111/hiv.13038
Transmitted HIV-1 drug resistance in a large international cohort using next-generation sequencing: results from the Strategic Timing of Antiretroviral Treatment (START) study
Abstract
Objectives: The aim of this analysis was to characterize transmitted drug resistance (TDR) in Strategic Timing of Antiretroviral Treatment (START) study participants by next-generation sequencing (NGS), a sensitive assay capable of detecting low-frequency variants.
Methods: Stored plasma from participants with entry HIV RNA > 1000 copies/mL were analysed by NGS (Illumina MiSeq). TDR was based on the WHO 2009 surveillance definition with the addition of reverse transcriptase (RT) mutations T215N and E138K, and integrase strand transfer inhibitor (INSTI) surveillance mutations (Stanford HIVdb). Drug resistance mutations (DRMs) detected at three thresholds are reported: > 2%, 5% and 20% of the viral population.
Results: Between 2009 and 2013, START enrolled 4684 antiretroviral therapy (ART)-naïve individuals in 35 countries. Baseline NGS data at study entry were available for 2902 participants. Overall prevalence rates of TDR using a detection threshold of 2%/5%/20% were 9.2%/5.6%/3.2% for nucleoside reverse transcriptase inhibitors (NRTIs), 9.2%/6.6%/4.9% for non-NRTIs, 11.4%/5.5%/2.4% for protease inhibitors (PIs) and 3.5%/1.6%/0.1% for INSTI DRMs and varied by geographic region. Using the 2% detection threshold, individual DRMs with the highest prevalence were: PI M46IL (5.5%), RT K103NS (3.5%), RT G190ASE (3.1%), T215ISCDVEN (2.5%), RT M41L (2.2%), RT K219QENR (1.7%) and PI D30N (1.6%). INSTI DRMs were detected almost exclusively below the 20% detection threshold, most commonly Y143H (0.4%), Q148R (0.4%) and T66I (0.4%).
Conclusions: Use of NGS in this study population resulted in the detection of a large proportion of low-level variants which would not have been detected by traditional Sanger sequencing. Global surveillance studies utilizing NGS should provide a more comprehensive assessment of TDR prevalence in different regions of the world.
Keywords: HIV; HIV drug resistance; antiretroviral therapy; next-generation sequencing.
© 2020 British HIV Association.
Conflict of interest statement
Figures
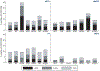

References
-
- Pillay D, Bhaskaran K, Jurriaans S et al. The impact of transmitted drug resistance on the natural history of HIV infection and response to first-line therapy. Aids 2006; 20: 21–8. - PubMed
-
- Little SJ, Holte S, Routy JP et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002; 347: 385–94. - PubMed
-
- Frentz D, Boucher C, Van De Vijver D. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev 2012; 14: 17–27. - PubMed
-
- Geretti AM. Epidemiology of antiretroviral drug resistance in drug-naive persons. Curr Opin Infect Dis 2007; 20: 22–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

