The anti-angiogenesis role of FBXW7 in diabetic retinopathy by facilitating the ubiquitination degradation of c-Myc to orchestrate the HDAC2
- PMID: 33369138
- PMCID: PMC7882985
- DOI: 10.1111/jcmm.16204
The anti-angiogenesis role of FBXW7 in diabetic retinopathy by facilitating the ubiquitination degradation of c-Myc to orchestrate the HDAC2
Abstract
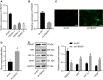
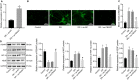
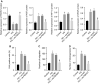
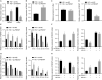
Diabetic retinopathy (DR) is the most prevalently occurring microvascular complication in diabetic patients that triggers severe visual impairments. The anti-angiogenesis role of FBXW7 has been identified in breast cancer. Therefore, this study intends to decipher the mechanism of FBXW7 in angiogenesis of DR. DR model was induced on mice using high-glucose (HG) and high-fat diet, and retinal microvascular endothelial cells (RMECs) isolated from normal mice were induced with HG, followed by evaluation of FBXW7, Ki67, HIF-1α and VEGF expression by immunofluorescence, immunohistochemistry or Western blot analysis. After gain- and loss-of-function assays in normal and DR mice, angiogenesis was assessed by CD31 fluorescence staining and Western blot analysis. After ectopic expression and silencing experiments in HG-induced RMECs, RMEC proliferation, migration and angiogenesis were, respectively, determined by EdU, Transwell and in vitro angiogenesis assays. The impact of FBXW7 on the ubiquitination of c-Myc was studied by cycloheximide chase assay and proteasome inhibition, and the binding of c-Myc to HDAC2 promoter by dual-luciferase reporter gene experiment. DR mice and HG-induced RMECs possessed down-regulated FBXW7 and up-regulated Ki67, HIF-1α and VEGF. Silencing FBXW7 enhanced angiogenesis in normal mouse retinal tissue, but overexpressing FBXW7 or silencing c-Myc diminished angiogenesis in DR mouse retinal tissue. Overexpressing FBXW7 or silencing c-Myc depressed proliferation, migration and angiogenesis in HG-induced RMECs. FBXW7 induced c-Myc ubiquitination degradation, and c-Myc augmented HDAC2 expression by binding to HDAC2 promoter. Conclusively, our data provided a novel sight of anti-angiogenesis role of FBXW7 in DR by modulating the c-Myc/HDAC2 axis.
Keywords: Angiogenesis; Diabetic retinopathy; FBXW7; HDAC2; HIF-1α; VEGF; c-Myc.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that they have no conflicts of interests.
Figures


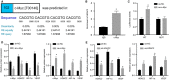
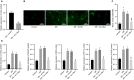

References
-
- Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8:337‐347. - PubMed
-
- Sabanayagam C, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140‐149. - PubMed
-
- Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156‐186. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

