Involvement of Müller Glial Autoinduction of TGF-β in Diabetic Fibrovascular Proliferation Via Glial-Mesenchymal Transition
- PMID: 33369638
- PMCID: PMC7774059
- DOI: 10.1167/iovs.61.14.29
Involvement of Müller Glial Autoinduction of TGF-β in Diabetic Fibrovascular Proliferation Via Glial-Mesenchymal Transition
Abstract
Purpose: Müller glial-mesenchymal transition (GMT) is reported as the fibrogenic mechanism promoted by TGF-β-SNAIL axis in Müller cells transdifferentiated into myofibroblasts. Here we show the multifaceted involvement of TGF-β in diabetic fibrovascular proliferation via Müller GMT and VEGF-A production.
Methods: Surgically excised fibrovascular tissues from the eyes of patients with proliferative diabetic retinopathy were processed for immunofluorescence analyses of TGF-β downstream molecules. Human Müller glial cells were used to evaluate changes in gene and protein expression with real-time quantitative PCR and ELISA, respectively. Immunoblot analyses were performed to detect TGF-β signal activation.
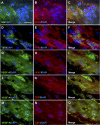
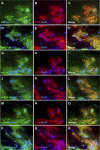
Results: Müller glial cells in patient fibrovascular tissues were immunopositive for GMT-related molecular markers, including SNAIL and smooth muscle protein 22, together with colocalization of VEGF-A and TGF-β receptors. In vitro administration of TGF-β1/2 upregulated TGFB1 and TGFB2, both of which were suppressed by inhibitors for nuclear factor-κB, glycogen synthase kinase-3, and p38 mitogen-activated protein kinase. Of the various profibrotic cytokines, TGF-β1/2 application exclusively induced Müller glial VEGFA mRNA expression, which was decreased by pretreatment with small interfering RNA for SMAD2 and inhibitors for p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Supporting these findings, TGF-β1/2 stimulation to Müller cells increased the phosphorylation of these intracellular signaling molecules, all of which were also activated in Müller glial cells in patient fibrovascular tissues.
Conclusions: This study underscored the significance of Müller glial autoinduction of TGF-β as a pathogenic cue to facilitate diabetic fibrovascular proliferation via TGF-β-driven GMT and VEGF-A-driven angiogenesis.
Conflict of interest statement
Disclosure:
Figures





References
-
- Bochaton-Piallat ML, Kapetanios AD, Donati G, Redard M, Gabbiani G, Pournaras CJ. TGF-beta1, TGF-beta receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Invest Ophthalmol Vis Sci. 2000; 41: 2336–2342. - PubMed
-
- Ishida S, Shinoda K, Kawashima S, Oguchi Y, Okada Y, Ikeda E. Coexpression of VEGF receptors VEGF-R2 and neuropilin-1 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000; 41: 1649–1656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

