Immunobridging efficacy of a tetravalent dengue vaccine against dengue and against hospitalized dengue from children/adolescents to adults in highly endemic countries
- PMID: 33369671
- PMCID: PMC8245293
- DOI: 10.1093/trstmh/traa154
Immunobridging efficacy of a tetravalent dengue vaccine against dengue and against hospitalized dengue from children/adolescents to adults in highly endemic countries
Abstract
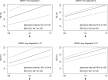
Background: The recombinant tetravalent live-attenuated dengue vaccine based on the YF 17D vaccine virus backbone (CYD-TDV) demonstrated vaccine efficacy (VE) against symptomatic, virologically confirmed dengue of any serotype from month 13 to month 25 (VCD-DENV-AnyM13→M25) in the CYD14 (2-14-y-olds) and CYD15 (9-16-y-olds) phase 3 trials. Fifty percent plaque reduction neutralization test (PRNT50) titers are a potential surrogate for immunobridging VE to adults.
Methods: Using PRNT50 calibration datasets, we applied immunobridging approaches using baseline and/or M13 PRNT50 titers to estimate VE against VCD-DENV-AnyM0→M25 and against hospitalized VCD (HVCD)-DENV-AnyM0→M72 in hypothetical 18-45-y-old and 46-50-y-old CYD14 and CYD15 cohorts.
Results: Baseline and M13 geometric mean PRNT50 titers were greater in 18-45-y-olds and in 46-50-y-olds vs 9-16-y-olds for most comparisons. Estimated VE (95% CIs against VCD-DENV-AnyM0→M25 ranged from 75.3% to 90.9% (52.5% to 100%) for 18-45-y-olds and 74.8% to 92.0% (53.4% to 100%) for 46-50-y-olds. Estimated VE (95% CIs) against HVCD-DENV-AnyM0→M72 ranged from 58.8% to 78.1% (40.9 to 98.9%) for 18-45-y-olds and 57.2% to 78.4% (40.5 to 97.6%) for 46-50-y-olds. Corresponding predictions among baseline-seropositive individuals yielded comparable or higher VE estimates.
Conclusions: VE M0→M25 against DENV-Any and VE against HVCD-DENV-AnyM0→M72 are both expected to be higher in 18-45 and 46-50-y-olds vs CYD14 and CYD15 9-16-y-olds.
Keywords: CYD-TDV; dengue vaccine; immunobridging; neutralizing antibodies; surrogate endpoint; vaccine efficacy.
© The Author(s) 2020. Published by Oxford University Press on behalf of Royal Society of Tropical Medicine and Hygiene.
Figures




References
-
- Shepard DS, Undurraga EA, Halasa YAet al. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16(8):935–41. - PubMed
-
- World Health Organization . Dengue and severe dengue. Available at https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue [accessed April 22, 2020].
-
- Capeding MR, Tran NH, Hadinegoro SRet al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384(9951):1358–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

