[11C]MODAG-001-towards a PET tracer targeting α-synuclein aggregates
- PMID: 33369690
- PMCID: PMC8113290
- DOI: 10.1007/s00259-020-05133-x
[11C]MODAG-001-towards a PET tracer targeting α-synuclein aggregates
Abstract
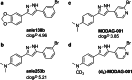
Purpose: Deposition of misfolded alpha-synuclein (αSYN) aggregates in the human brain is one of the major hallmarks of synucleinopathies. However, a target-specific tracer to detect pathological aggregates of αSYN remains lacking. Here, we report the development of a positron emission tomography (PET) tracer based on anle138b, a compound shown to have therapeutic activity in animal models of neurodegenerative diseases.
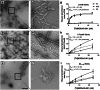
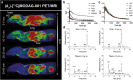
Methods: Specificity and selectivity of [3H]MODAG-001 were tested in in vitro binding assays using recombinant fibrils. After carbon-11 radiolabeling, the pharmacokinetic and metabolic profile was determined in mice. Specific binding was quantified in rats, inoculated with αSYN fibrils and using in vitro autoradiography in human brain sections of Lewy body dementia (LBD) cases provided by the Neurobiobank Munich (NBM).
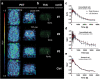
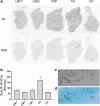
Results: [3H]MODAG-001 revealed a very high affinity towards pure αSYN fibrils (Kd = 0.6 ± 0.1 nM) and only a moderate affinity to hTau46 fibrils (Kd = 19 ± 6.4 nM) as well as amyloid-β1-42 fibrils (Kd = 20 ± 10 nM). [11C]MODAG-001 showed an excellent ability to penetrate the mouse brain. Metabolic degradation was present, but the stability of the parent compound improved after selective deuteration of the precursor. (d3)-[11C]MODAG-001 binding was confirmed in fibril-inoculated rat striata using in vivo PET imaging. In vitro autoradiography showed no detectable binding to aggregated αSYN in human brain sections of LBD cases, most likely, because of the low abundance of aggregated αSYN against background protein.
Conclusion: MODAG-001 provides a promising lead structure for future compound development as it combines a high affinity and good selectivity in fibril-binding assays with suitable pharmacokinetics and biodistribution properties.
Keywords: Alpha-synuclein; PET imaging; Parkinson’s disease; Tracer development.
Conflict of interest statement
A patent has been filed.
Armin Giese, Felix Schmidt, Daniel Weckbecker, Andrei Leonov, and Sergey Ryazanov are employed by MODAG GmbH, which retains ownership of MODAG-001, and Armin Giese and Christian Griesinger are shareholders of MODAG GmbH.
Figures

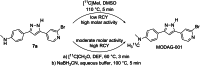

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

