Post-exercise intramuscular O2 supply is tightly coupled with a higher proximal-to-distal ATP synthesis rate in human tibialis anterior
- PMID: 33369737
- PMCID: PMC7986184
- DOI: 10.1113/JP280771
Post-exercise intramuscular O2 supply is tightly coupled with a higher proximal-to-distal ATP synthesis rate in human tibialis anterior
Abstract
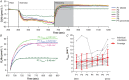
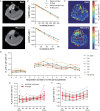
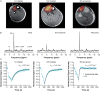
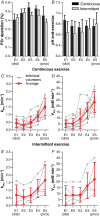
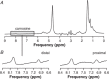
Key points: The post-exercise recovery of phosphocreatine, a measure of the oxidative capacity of muscles, as assessed by 31 P MR spectroscopy, shows a striking increase from distal to proximal along the human tibialis anterior muscle. To investigate why this muscle exhibits a greater oxidative capacity proximally, we tested whether the spatial variation in phosphocreatine recovery rate is related to oxygen supply, muscle fibre type or type of exercise. We revealed that oxygen supply also increases from distal to proximal along the tibialis anterior, and that it strongly correlated with phosphocreatine recovery. Carnosine level, a surrogate measure for muscle fibre type was not different between proximal and distal, and type of exercise did not affect the gradient in phosphocreatine recovery rate. Taken together, the findings of this study suggest that the post-exercise spatial gradients in oxygen supply and phosphocreatine recovery are driven by a higher intrinsic mitochondrial oxidative capacity proximally.
Abstract: Phosphorus magnetic resonance spectroscopy (31 P MRS) of human tibialis anterior (TA) revealed a strong proximo-distal gradient in the post-exercise phosphocreatine (PCr) recovery rate constant (kPCr ), a measure of muscle oxidative capacity. The aim of this study was to investigate whether this kPCr gradient is related to O2 supply, resting phosphorylation potential, muscle fibre type, or type of exercise. Fifteen male volunteers performed continuous isometric ankle dorsiflexion at 30% maximum force until exhaustion. At multiple locations along the TA, we measured the oxidative PCr resynthesis rate (VPCr = kPCr × PCr depletion) by 31 P MRS, the oxyhaemoglobin recovery rate constant (kO2Hb ) by near infrared spectroscopy, and muscle perfusion with MR intravoxel incoherent motion imaging. The kO2Hb , kPCr , VPCr and muscle perfusion depended on measurement location (P < 0.001, P < 0.001, P = 0.032 and P = 0.003, respectively), all being greater proximally. The kO2Hb and muscle perfusion correlated with kPCr (r = 0.956 and r = 0.852, respectively) and VPCr (r = 0.932 and r = 0.985, respectively), the latter reflecting metabolic O2 consumption. Resting phosphorylation potential (PCr/inorganic phosphate) was also higher proximally (P < 0.001). The surrogate for fibre type, carnosine content measured by 1 H MRS, did not differ between distal and proximal TA (P = 0.884). Performing intermittent exercise to avoid exercise ischaemia, still led to larger kPCr proximally than distally (P = 0.013). In conclusion, the spatial kPCr gradient is strongly associated with the spatial variation in O2 supply. It cannot be explained by exercise-induced ischaemia nor by fibre type. Our findings suggest it is driven by a higher proximal intrinsic mitochondrial oxidative capacity, apparently to support contractile performance of the TA.
Keywords: 31P magnetic resonance spectroscopy; magnetic resonance imaging; oxidative metabolism; phosphocreatine recovery; skeletal muscle.
© 2020 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures


References
-
- Arnold DL, Taylor DJ & Radda GK (1985). Investigation of human mitochondrial myopathies by phosphorus magnetic resonance spectroscopy. Ann Neurol 18, 189–196. - PubMed
-
- Bendahan D, Confort‐Gouny S, Kozak‐Reiss G & Cozzone PJ (1990). Heterogeneity of metabolic response to muscular exercise in humans. New criteria of invariance defined by in vivo phosphorus‐31 NMR spectroscopy. FEBS Lett 272, 155–158. - PubMed
-
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J & Laval‐Jeantet M (1988). Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168, 497–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

