Frontiers in Multiscale Modeling of Photoreceptor Proteins
- PMID: 33369749
- PMCID: PMC9185909
- DOI: 10.1111/php.13372
Frontiers in Multiscale Modeling of Photoreceptor Proteins
Abstract
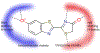
This perspective article highlights the challenges in the theoretical description of photoreceptor proteins using multiscale modeling, as discussed at the CECAM workshop in Tel Aviv, Israel. The participants have identified grand challenges and discussed the development of new tools to address them. Recent progress in understanding representative proteins such as green fluorescent protein, photoactive yellow protein, phytochrome, and rhodopsin is presented, along with methodological developments.
© 2021 American Society for Photobiology.
Figures
















References
-
- Van Der Horst MA and Hellingwerf KJ (2004) Photoreceptor proteins, “star actors of modern times”: A review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc. Chem. Res 37(1), 13–20. - PubMed
-
- Zimmer M (2002) Green fluorescent protein (GFP): applications, structure, and related photophysical behavior. Chem. Rev 102(3), 759–781. - PubMed
-
- Reis JM and Andrew Woolley G (2016) Photo control of protein function using photoactive yellow protein. In: Methods in Molecular Biology, Vol. 1408, pp. 79–92.Humana Press Inc. - PubMed
-
- Meech SR (2009) Excited state reactions in fluorescent proteins. Chem. Soc. Rev 38(10), 2922–2934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

