Craniofacial and Long Bone Development in the Context of Distraction Osteogenesis
- PMID: 33370054
- PMCID: PMC7773036
- DOI: 10.1097/PRS.0000000000007451
Craniofacial and Long Bone Development in the Context of Distraction Osteogenesis
Abstract
Background: Bone retains regenerative potential into adulthood, and surgeons harness this plasticity during distraction osteogenesis. The underlying biology governing bone development, repair, and regeneration is divergent between the craniofacial and appendicular skeleton. Each type of bone formation is characterized by unique molecular signaling and cellular behavior. Recent discoveries have elucidated the cellular and genetic processes underlying skeletal development and regeneration, providing an opportunity to couple biological and clinical knowledge to improve patient care.
Methods: A comprehensive literature review of basic and clinical literature regarding craniofacial and long bone development, regeneration, and distraction osteogenesis was performed.
Results: The current understanding in craniofacial and long bone development and regeneration is discussed, and clinical considerations for the respective distraction osteogenesis procedures are presented.
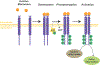
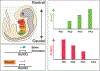
Conclusions: Distraction osteogenesis is a powerful tool to regenerate bone and thus address a number of craniofacial and appendicular skeletal deficiencies. The molecular mechanisms underlying bone regeneration, however, remain elusive. Recent work has determined that embryologic morphogen gradients constitute important signals during regeneration. In addition, striking discoveries have illuminated the cellular processes underlying mandibular regeneration during distraction osteogenesis, showing that skeletal stem cells reactivate embryologic neural crest transcriptomic processes to carry out bone formation during regeneration. Furthermore, innovative adjuvant therapies to complement distraction osteogenesis use biological processes active in embryogenesis and regeneration. Additional research is needed to further characterize the underlying cellular mechanisms responsible for improved bone formation through adjuvant therapies and the role skeletal stem cells play during regeneration.
Copyright © 2020 by the American Society of Plastic Surgeons.
Figures


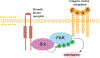
References
-
- Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–743. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

