Erythrocytosis: genes and pathways involved in disease development
- PMID: 33370224
- PMCID: PMC8580782
- DOI: 10.2450/2020.0197-20
Erythrocytosis: genes and pathways involved in disease development
Abstract
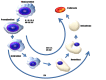
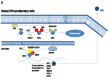
Erythrocytosis is a blood disorder characterised by an increased red blood cell mass. The most common causes of erythrocytosis are acquired and caused by diseases and conditions that are accompanied by hypoxaemia or overproduction of erythropoietin. More rarely, erythrocytosis has a known genetic background, such as for polycythaemia vera and familial erythrocytosis. The majority of cases of polycythaemia vera are associated with acquired variants in JAK2, while familial erythrocytosis is a group of congenital disorders. Familial erythrocytosis type 1 is associated with hypersensitivity to erythropoietin (variants in EPOR), types 2-5 with defects in oxygen-sensing pathways (variants in VHL, EGLN1, EPAS1, EPO), and types 6-8 with an increased affinity of haemoglobin for oxygen (variants in HBB, HBA1, HBA2, BPGM). Due to a heterogenic genetic background, the causes of disease are not fully discovered and in more than 70% of patients the condition remains labelled idiopathic.The transfer of next-generation sequencing into clinical practice is becoming a reality enabling detection of various variants in a single rapid test. In this review, we describe the current research on erythrocytosis gene variants and the mechanisms associated with disease development, along with the currently used diagnostic tests.
Conflict of interest statement
The Authors declare that they have no conflicts of interest.
Figures


References
-
- Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet. 2000;1:57–64. - PubMed
-
- Bento C, Percy MJ, Gardie B, et al. Genetic basis of congenital erythrocytosis: mutation update and online databases. Hum Mutat. 2014;35:15–26. - PubMed
-
- Debeljak N, Lazarevič J, Miskič D, et al. Characterization of erythrocytosis and a proposed diagnostic algorithm in Slovenia. Zdrav Vest. 2019;88:5–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
