Antileishmanial activity of synthetic analogs of the naturally occurring quinolone alkaloid N-methyl-8-methoxyflindersin
- PMID: 33370295
- PMCID: PMC7769561
- DOI: 10.1371/journal.pone.0243392
Antileishmanial activity of synthetic analogs of the naturally occurring quinolone alkaloid N-methyl-8-methoxyflindersin
Abstract
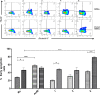
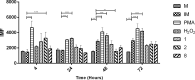
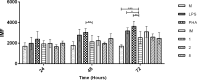
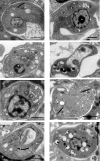
Leishmaniasis is a neglected, parasitic tropical disease caused by an intracellular protozoan from the genus Leishmania. Quinoline alkaloids, secondary metabolites found in plants from the Rutaceae family, have antiparasitic activity against Leishmania sp. N-methyl-8-methoxyflindersin (1), isolated from the leaves of Raputia heptaphylla and also known as 7-methoxy-2,2-dimethyl-2H,5H,6H-pyran[3,2-c]quinolin-5-one, shows antiparasitic activity against Leishmania promastigotes and amastigotes. This study used in silico tools to identify synthetic quinoline alkaloids having structure similar to that of compound 1 and then tested these quinoline alkaloids for their in vitro antiparasitic activity against Leishmania (Viannia) panamensis, in vivo therapeutic response in hamsters suffering from experimental cutaneous leishmaniasis (CL), and ex vivo immunomodulatory potential in healthy donors' human peripheral blood (monocyte)-derived macrophages (hMDMs). Compounds 1 (natural), 2 (synthetic), and 8 (synthetic) were effective against intracellular promastigotes (9.9, 3.4, and 1.6 μg/mL medial effective concentration [EC50], respectively) and amastigotes (5.07, 7.94, and 1.91 μg/mL EC50, respectively). Compound 1 increased nitric oxide production in infected hMDMs and triggered necrosis-related ultrastructural alterations in intracellular amastigotes, while compound 2 stimulated oxidative breakdown in hMDMs and caused ultrastructural alterations in the parasite 4 h posttreatment, and compound 8 failed to induce macrophage modulation but selectively induced apoptosis of infected hMDMs and alterations in the intracellular parasite ultrastructure. In addition, synthetic compounds 2 and 8 improved the health of hamsters suffering from experimental CL, without evidence of treatment-associated adverse toxic effects. Therefore, synthetic compounds 2 and 8 are potential therapeutic candidates for topical treatment of CL.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
seco-limonoids and quinoline alkaloids from Raputia heptaphylla and their antileishmanial activity.Chem Pharm Bull (Tokyo). 2011;59(7):855-9. doi: 10.1248/cpb.59.855. Chem Pharm Bull (Tokyo). 2011. PMID: 21720036
-
Leishmanicidal effect of Spiranthera odoratíssima (Rutaceae) and its isolated alkaloid skimmianine occurs by a nitric oxide dependent mechanism.Parasitology. 2011 Sep;138(10):1224-33. doi: 10.1017/S0031182011001168. Epub 2011 Aug 3. Parasitology. 2011. PMID: 21810308
-
Profiling gene expression of antimony response genes in Leishmania (Viannia) panamensis and infected macrophages and its relationship with drug susceptibility.Acta Trop. 2017 Dec;176:355-363. doi: 10.1016/j.actatropica.2017.08.017. Epub 2017 Aug 24. Acta Trop. 2017. PMID: 28843396 Free PMC article.
-
Antileishmanial Activities of Medicinal Herbs and Phytochemicals In Vitro and In Vivo: An Update for the Years 2015 to 2021.Molecules. 2022 Nov 4;27(21):7579. doi: 10.3390/molecules27217579. Molecules. 2022. PMID: 36364404 Free PMC article. Review.
-
Natural products derived steroids as potential anti-leishmanial agents; disease prevalence, underlying mechanisms and future perspectives.Steroids. 2023 May;193:109196. doi: 10.1016/j.steroids.2023.109196. Epub 2023 Feb 9. Steroids. 2023. PMID: 36764565 Review.
Cited by
-
Efficacy Evaluation of 10-Hydroxy Chondrofoline and Tafenoquine against Leishmania tropica (HTD7).Pharmaceuticals (Basel). 2022 Aug 15;15(8):1005. doi: 10.3390/ph15081005. Pharmaceuticals (Basel). 2022. PMID: 36015153 Free PMC article.
-
Evolution of the Quinoline Scaffold for the Treatment of Leishmaniasis: A Structural Perspective.Pharmaceuticals (Basel). 2024 Feb 22;17(3):285. doi: 10.3390/ph17030285. Pharmaceuticals (Basel). 2024. PMID: 38543071 Free PMC article. Review.
-
The quinoline framework and related scaffolds in natural products with anti-Leishmania properties.Front Chem. 2025 Apr 25;13:1571067. doi: 10.3389/fchem.2025.1571067. eCollection 2025. Front Chem. 2025. PMID: 40351526 Free PMC article. Review.
-
Plant-derived products as anti-leishmanials which target mitochondria: a review.Expert Rev Mol Med. 2025 Mar 26;27:e15. doi: 10.1017/erm.2025.8. Expert Rev Mol Med. 2025. PMID: 40134281 Free PMC article. Review.
-
Therapeutic Activity of a Topical Formulation Containing 8-Hydroxyquinoline for Cutaneous Leishmaniasis.Pharmaceutics. 2023 Nov 8;15(11):2602. doi: 10.3390/pharmaceutics15112602. Pharmaceutics. 2023. PMID: 38004580 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

