Ischemia and reperfusion injury to mitochondria and cardiac function in donation after circulatory death hearts- an experimental study
- PMID: 33370296
- PMCID: PMC7769461
- DOI: 10.1371/journal.pone.0243504
Ischemia and reperfusion injury to mitochondria and cardiac function in donation after circulatory death hearts- an experimental study
Abstract
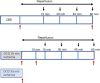
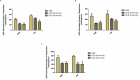
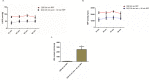
The ultimate treatment for patients with end-stage heart failure is heart transplantation. The number of donor hearts which are primarily procured from donation after brain death (DBD) donors is limited, but donation after circulatory death (DCD) donor hearts can increase the heart donor pool. However, ischemia and reperfusion injuries associated with the DCD process causes myocardial damage, limiting the use of DCD hearts in transplantation. Addressing this problem is critical in the exploration of DCD hearts as suitable donor hearts for transplantation. In this study, rat hearts were procured following the control beating-heart donor (CBD) or DCD donation process. Changes in mitochondria and cardiac function from DCD hearts subjected to 25 or 35 minutes of ischemia followed by 60 minutes of reperfusion were compared to CBD hearts. Following ischemia, rates of oxidative phosphorylation and calcium retention capacity were progressively impaired in DCD hearts compared to CBD hearts. Reperfusion caused additional mitochondrial dysfunction in DCD hearts. Developed pressure, inotropy and lusitropy, were significantly reduced in DCD hearts compared to CBD hearts. We, therefore, suggest that interventional strategies targeted before the onset of ischemia and at reperfusion could protect mitochondria, thus potentially making DCD hearts suitable for heart transplantation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Mozzafarian D, Benjamin EJ, Go AS, et al. On behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016; 133: e38–e360. 10.1161/CIR.0000000000000350 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

