Observational study on time on treatment with abiraterone and enzalutamide
- PMID: 33370378
- PMCID: PMC7769419
- DOI: 10.1371/journal.pone.0244462
Observational study on time on treatment with abiraterone and enzalutamide
Abstract
Introduction: The aim of this study was to assess time on treatment with abiraterone and enzalutamide, two androgen receptor targeted (ART) drugs, the impact on time on treatment of time interval without drug supply between prescription fillings, and adherence to treatment.
Material and methods: By use of data from The National Prostate Cancer Register, The Prescribed Drug Registry and the Patient Registry, time on treatment with the abiraterone and enzalutamide was analyzed in all men with castration resistant prostate cancer (CRPC) in Sweden 2015-2019. Three time intervals between consecutive fillings, i.e. time without drug supply, were assessed. Adherence to the treatment was evaluated by use of the Medication Possession Ratio. Kaplan Meier analysis and multivariable Cox regression model were used to assess factors affecting time on treatment.
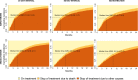
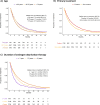
Results: Between January 2015 and October 2019, 1803 men filled a prescription for abiraterone and 4 534 men filled a prescription for enzalutamide. With a time interval of 30 days or less between two fillings, median time on treatment was 4.9 months (IQR 2.6-11.7) for abiraterone and 8.0 months (IQR 3.6-16.4) for enzalutamide. In sensitivity analyses, allowing for no more than 14 days without drug supply between fillings, median time on treatment was 3.9 months (IQR 2.1-9.0) for abiraterone and 5.9 months (IQR 2.8-12.1) for enzalutamide. Allowing for any time period without drug between fillings, median time on treatment was 5.7 months (IQR 2.7-14.0) for abiraterone and 9.8 months (IQR 4.4-21.0) for enzalutamide. Adherence to treatment was above 90% for both drugs.
Conclusion: Time on treatment with abiraterone and enzalutamide was shorter in clinical practice than in randomized controlled trials and varied almost two-fold with time interval without drug. Adherence to treatment was high. The main limitation of our study was the lack of data on use of chemotherapy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13: 983–992. 10.1016/S1470-2045(12)70379-0 - DOI - PubMed
-
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PFA, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16: 152–160. 10.1016/S1470-2045(14)71205-7 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

