Comorbidities and complications in adults with pyruvate kinase deficiency
- PMID: 33370479
- PMCID: PMC7985869
- DOI: 10.1111/ejh.13572
Comorbidities and complications in adults with pyruvate kinase deficiency
Abstract
Objectives: Pyruvate kinase (PK) deficiency is caused by PKLR gene mutations, leading to defective red blood cell glycolysis and hemolytic anemia. Rates of comorbidities and complications by transfusion history and relative to the general population remain poorly quantified.
Methods: Data for patients aged ≥ 18 years with two confirmed PKLR mutations were obtained from the PK deficiency Natural History Study (NCT02053480). Frequencies of select conditions were compared with an age- and sex-matched cohort from a general insured US population without PK deficiency.
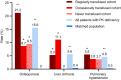
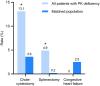
Results: Compared with the matched population (n = 1220), patients with PK deficiency (n = 122) had significantly higher lifetime rates of osteoporosis, liver cirrhosis, and pulmonary hypertension; splenectomy and cholecystectomy rates were also significantly higher in the 8 years before the index date. Sixty-five (53.3%) patients with PK deficiency were classified as regularly transfused, 30 (24.6%) as occasionally transfused, and 27 (22.1%) as never transfused. Regularly transfused patients were significantly more likely than never transfused patients to have had splenectomy, cholecystectomy, and/or thrombosis. Liver iron overload was reported in 62% of patients and occurred regardless of transfusion cohort.
Conclusions: Even never transfused patients with PK deficiency had higher rates of select comorbidities and complications than individuals without PK deficiency.
Keywords: blood transfusion; comorbidity; pyruvate kinase deficiency.
© 2020 Agios Pharmaceuticals, Inc. European Journal of Haematology Published by John Wiley & Sons Ltd.
Conflict of interest statement
ANB and YY are employees of and hold stock in Agios. EH received funding for this research from Agios through a contract with IBM Watson Health. EJvB has received research support from Agios, Bayer, Mechatronics, and Novartis; and has served as a consultant for Agios. HA‐S reports research support from Agios, Amgen, and Dova; and has served as a consultant for Agios and Dova. WB has received research support from Alexion and Novartis; has served on advisory boards for Agios, Alexion, Bioverativ, and Incyte; and has served on speaker bureaus for Agios, Alexion, and Novartis. SWE is a consultant for Agios. BG is a research investigator and consultant for Agios. HMY has served as a consultant for Agios, Bayer, Novo Nordisk, Octapharma, and Takeda; and has served on speaker bureaus for Bayer and Takeda. SC has served as a consultant and on advisory boards for Agios and Alexion. KHMK has received honoraria from and is a consultant for Agios, Apellis, bluebird bio, Celgene, and Pfizer; and has served on a data safety monitoring board for Bioverativ. EJN has served as a consultant and on advisory boards for Agios, Celgene, and Genentech; has served as a consultant for Pfizer; has served on advisory boards for Baxalta/Shire (now Takeda) and Novartis; has served as a consultant, on advisory boards, and received honoraria from Octapharma; has served on advisory boards and received honoraria from Novo Nordisk; and has served on data monitoring committees for ApoPharma, Acceleron, Bayer, and Imara. MV has served as a consultant for Vertex. SS has served as a consultant for Acceleron, Agios, bluebird bio, and Celgene/BMS; has served on a clinical trial steering committee for CRISPR/Vertex; and participated in clinical trials with Celgene/BMS, Dispersol, La Jolla, and Terumo. RFG has served on advisory boards for Dova; has served on advisory boards and received research funding from Agios; and has received research funding from Novartis and Pfizer. The remaining authors declare no competing financial interests.
Figures
References
-
- Zanella A, Fermo E, Bianchi P, Chiarelli LR, Valentini G. Pyruvate kinase deficiency: the genotype‐phenotype association. Blood Rev. 2007;21(4):217‐231. - PubMed
-
- van Wijk R, van Solinge WW. The energy‐less red blood cell is lost: erythrocyte enzyme abnormalities of glycolysis. Blood. 2005;106(13):4034‐4042. - PubMed
-
- Nathan DG, Oski FA, Miller DR, Gardner FH. Life‐span and organ sequestration of the red cells in pyruvate kinase deficiency. N Engl J Med. 1968;278(2):73‐81. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

