RAS inhibition in resident fibroblast biology
- PMID: 33370581
- PMCID: PMC7878352
- DOI: 10.1016/j.cellsig.2020.109903
RAS inhibition in resident fibroblast biology
Abstract
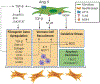
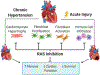
Angiotensin II (Ang II) is a primary mediator of profibrotic signaling in the heart and more specifically, the cardiac fibroblast. Ang II-mediated cardiomyocyte hypertrophy in combination with cardiac fibroblast proliferation, activation, and extracellular matrix production compromise cardiac function and increase mortality in humans. Profibrotic actions of Ang II are mediated by increasing production of fibrogenic mediators (e.g. transforming growth factor beta, scleraxis, osteopontin, and periostin), recruitment of immune cells, and via increased reactive oxygen species generation. Drugs that inhibit Ang II production or action, collectively referred to as renin angiotensin system (RAS) inhibitors, are first line therapeutics for heart failure. Moreover, transient RAS inhibition has been found to persistently alter hypertensive cardiac fibroblast responses to injury providing a useful tool to identify novel therapeutic targets. This review summarizes the profibrotic actions of Ang II and the known impact of RAS inhibition on cardiac fibroblast phenotype and cardiac remodeling.
Keywords: Angiotensin II; Angiotensin converting enzyme inhibitor; Angiotensin receptor antagonist; Fibroblast; Fibrosis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures

References
-
- Müller DN, Fischli W, Clozel J-P, Hilgers KF, Bohlender J, Ménard J, Busjahn A, Ganten D, Luft FC. Local Angiotensin II Generation in the Rat Heart. Circulation research. 1998;82(1):13–20. - PubMed
-
- Heller LJ, Opsahl JA, Wernsing SE, Saxena R, Katz SA. Myocardial and plasma renin-angiotensinogen dynamics during pressure-induced cardiac hypertrophy. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1998;274(3):R849–R856. - PubMed
-
- Passier RC, Smits JF, Verluyten MJ, Daemen MJ. Expression and localization of renin and angiotensinogen in rat heart after myocardial infarction. Am J Physiol. 1996;271(3 Pt 2):H1040–1048. - PubMed
-
- Sun Y, Zhang J, Zhang JQ, Weber KT. Renin expression at sites of repair in the infarcted rat heart. Journal of molecular and cellular cardiology. 2001;33(5):995–1003. - PubMed
-
- Yamada H, Fabris B, Allen A, Jackson B, Johnston CI, Mendelsohn A. Localization of angiotensin converting enzyme in rat heart. Circulation research. 1991;68(1):141–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

