Roles and Mechanisms of the Long Noncoding RNAs in Cervical Cancer
- PMID: 33371204
- PMCID: PMC7766288
- DOI: 10.3390/ijms21249742
Roles and Mechanisms of the Long Noncoding RNAs in Cervical Cancer
Abstract
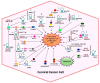
Cervical cancer (CC) continues to be one of the leading causes of death for women across the world. Although it has been determined that papillomavirus infection is one of the main causes of the etiology of the disease, genetic and epigenetic factors are also required for its progression. Among the epigenetic factors are included the long noncoding RNAs (lncRNAs), transcripts of more than 200 nucleotides (nt) that generally do not code for proteins and have been associated with diverse functions such as the regulation of transcription, translation, RNA metabolism, as well as stem cell maintenance and differentiation, cell autophagy and apoptosis. Recently, studies have begun to characterize the aberrant regulation of lncRNAs in CC cells and tissues, including Homeobox transcript antisense RNA (HOTAIR), H19, Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), Cervical Carcinoma High-Expressed 1 (CCHE1), Antisense noncoding RNA in the inhibitors of cyclin-dependent kinase 4 (ANRIL), Growth arrest special 5 (GAS5) and Plasmacytoma variant translocation 1 (PVT1). They have been associated with several disease-related processes such as cell growth, cell proliferation, cell survival, metastasis and invasion as well as therapeutic resistance, and are novel potential biomarkers for diagnosis and prognosis in CC. In this review, we summarize the current literature regarding the knowledge we have about the roles and mechanisms of the lncRNAs in cervical neoplasia.
Keywords: biological function; cervical cancer; epigenetics; long noncoding RNAs; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Draft Global Strategy towards Eliminating Cervical Cancer as a Public Health Problem. [(accessed on 27 September 2020)]; Available online: https://www.who.int/publications/m/item/draft-global-strategy-towards-el....

