Silicon Nanowire Field-Effect Transistor as Biosensing Platforms for Post-Translational Modification
- PMID: 33371301
- PMCID: PMC7767353
- DOI: 10.3390/bios10120213
Silicon Nanowire Field-Effect Transistor as Biosensing Platforms for Post-Translational Modification
Abstract
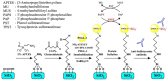
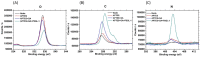
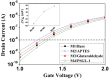
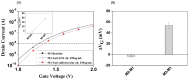
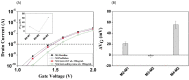
Protein tyrosine sulfation (PTS), a vital post-translational modification, facilitates protein-protein interactions and regulates many physiological and pathological responses. Monitoring PTS has been difficult owing to the instability of sulfated proteins and the lack of a suitable method for detecting the protein sulfate ester. In this study, we combined an in situ PTS system with a high-sensitivity polysilicon nanowire field-effect transistor (pSNWFET)-based sensor to directly monitor PTS formation. A peptide containing the tyrosine sulfation site of P-selectin glycoprotein ligand (PSGL)-1 was immobilized onto the surface of the pSNWFET by using 3-aminopropyltriethoxysilane and glutaraldehyde as linker molecules. A coupled enzyme sulfation system consisting of tyrosylprotein sulfotransferase and phenol sulfotransferase was used to catalyze PTS of the immobilized PSGL-1 peptide. Enzyme-catalyzed sulfation of the immobilized peptide was readily observed through the shift of the drain current-gate voltage curves of the pSNWFET before and after PTS. We expect that this approach can be developed as a next generation biochip for biomedical research and industries.
Keywords: polycrystalline silicon nanowire field-effect transistor (pSNWFET); post-translational modifications (PTMs); protein tyrosine sulfation (PTS); protein–protein interaction.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




Similar articles
-
Preparation of Silicon Nanowire Field-effect Transistor for Chemical and Biosensing Applications.J Vis Exp. 2016 Apr 21;(110):53660. doi: 10.3791/53660. J Vis Exp. 2016. PMID: 27167162 Free PMC article.
-
Tyrosine sulfation as a protein post-translational modification.Molecules. 2015 Jan 28;20(2):2138-64. doi: 10.3390/molecules20022138. Molecules. 2015. PMID: 25635379 Free PMC article. Review.
-
Fluorescence assay for protein post-translational tyrosine sulfation.Anal Bioanal Chem. 2013 Feb;405(4):1425-9. doi: 10.1007/s00216-012-6540-3. Epub 2012 Nov 18. Anal Bioanal Chem. 2013. PMID: 23161068
-
Tyrosine sulfation enhances but is not required for PSGL-1 rolling adhesion on P-selectin.Biophys J. 2001 Oct;81(4):2001-9. doi: 10.1016/S0006-3495(01)75850-X. Biophys J. 2001. PMID: 11566773 Free PMC article.
-
Tyrosine sulfation: an increasingly recognised post-translational modification of secreted proteins.N Biotechnol. 2009 Jun;25(5):299-317. doi: 10.1016/j.nbt.2009.03.011. N Biotechnol. 2009. PMID: 19658209 Review.
Cited by
-
Ultrasensitive Silicon Nanowire Biosensor with Modulated Threshold Voltages and Ultra-Small Diameter for Early Kidney Failure Biomarker Cystatin C.Biosensors (Basel). 2023 Jun 13;13(6):645. doi: 10.3390/bios13060645. Biosensors (Basel). 2023. PMID: 37367010 Free PMC article.
-
Silicon Nanowires Length and Numbers Dependence on Sensitivity of the Field-Effect Transistor Sensor for Hepatitis B Virus Surface Antigen Detection.Biosensors (Basel). 2022 Feb 12;12(2):115. doi: 10.3390/bios12020115. Biosensors (Basel). 2022. PMID: 35200375 Free PMC article.
-
Silicon Nanowire Field-Effect Transistor as Label-Free Detection of Hepatitis B Virus Proteins with Opposite Net Charges.Biosensors (Basel). 2021 Nov 10;11(11):442. doi: 10.3390/bios11110442. Biosensors (Basel). 2021. PMID: 34821658 Free PMC article.
-
Fabrication of Silicon Nanowires by Metal-Assisted Chemical Etching Combined with Micro-Vibration.Materials (Basel). 2023 Aug 5;16(15):5483. doi: 10.3390/ma16155483. Materials (Basel). 2023. PMID: 37570187 Free PMC article.
-
Recent Advances of Field-Effect Transistor Technology for Infectious Diseases.Biosensors (Basel). 2021 Apr 2;11(4):103. doi: 10.3390/bios11040103. Biosensors (Basel). 2021. PMID: 33918325 Free PMC article. Review.
References
-
- Huang B.-Y., Chen P.-C., Chen B.-H., Wang C.-C., Liu H.-F., Chen Y.-Z., Chen C.-S., Yang Y.-S. High-throughput screening of sulfated proteins by using a genome-wide proteome microarray and protein tyrosine sulfation system. Anal. Chem. 2017;89:3278–3284. doi: 10.1021/acs.analchem.6b02853. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

