Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue
- PMID: 33371306
- PMCID: PMC7767389
- DOI: 10.3390/ijms21249759
Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue
Abstract
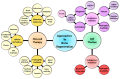
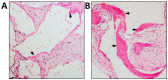
There has been an escalation in reports over the last decade examining the efficacy of bone marrow derived mesenchymal stem/stromal cells (BMSC) in bone tissue engineering and regenerative medicine-based applications. The multipotent differentiation potential, myelosupportive capacity, anti-inflammatory and immune-modulatory properties of BMSC underpins their versatile nature as therapeutic agents. This review addresses the current limitations and challenges of exogenous autologous and allogeneic BMSC based regenerative skeletal therapies in combination with bioactive molecules, cellular derivatives, genetic manipulation, biocompatible hydrogels, solid and composite scaffolds. The review highlights the current approaches and recent developments in utilizing endogenous BMSC activation or exogenous BMSC for the repair of long bone and vertebrae fractures due to osteoporosis or trauma. Current advances employing BMSC based therapies for bone regeneration of craniofacial defects is also discussed. Moreover, this review discusses the latest developments utilizing BMSC therapies in the preclinical and clinical settings, including the treatment of bone related diseases such as Osteogenesis Imperfecta.
Keywords: biomaterials; bone marrow mesenchymal stem cells; bone marrow microenvironment; scaffolds; skeletal tissue regeneration; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Arthur A., Zannettino A., Gronthos S. Multipotential mesenchymal stromal/stem cells in skeletal tissue repair. In: Rajkumar Rajendram V.R.P., Patel V., editors. Stem Cells and Bone Tissue. 1st ed. CRC Press; Boca Raton, FL, USA: 2013. pp. 82–102.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

