Comparison of the Zebrafish Embryo Toxicity Assay and the General and Behavioral Embryo Toxicity Assay as New Approach Methods for Chemical Screening
- PMID: 33371320
- PMCID: PMC7767334
- DOI: 10.3390/toxics8040126
Comparison of the Zebrafish Embryo Toxicity Assay and the General and Behavioral Embryo Toxicity Assay as New Approach Methods for Chemical Screening
Abstract
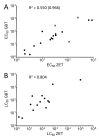
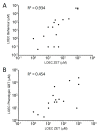
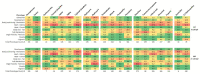
The movement away from mammalian testing of potential toxicants and new chemical entities has primarily led to cell line testing and protein-based assays. However, these assays may not yet be sufficient to properly characterize the toxic potential of a chemical. The zebrafish embryo model is widely recognized as a potential new approach method for chemical testing that may provide a bridge between cell and protein-based assays and mammalian testing. The Zebrafish Embryo Toxicity (ZET) model is increasingly recognized as a valuable toxicity testing platform. The ZET assay focuses on the early stages of embryo development and is considered a more humane model compared to adult zebrafish testing. A complementary model has been developed that exposes larvae to toxicants at a later time point during development where body patterning has already been established. Here we compare the toxicity profiles of 20 compounds for this General and Behavioral Toxicity (GBT) assay to the ZET assay. The results show partially overlapping toxicity profiles along with unique information provided by each assay. It appears from this work that these two assays applied together can strengthen the use of zebrafish embryos/larvae as standard toxicity testing models.
Keywords: developmental period; phenotype; toxicity assay; zebrafish larvae.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Caballero M.V., Candiracci M. Zebrafish as Toxicological model for screening and recapitulate human diseases. J. Unexplored Med. Data. 2018;3:4. doi: 10.20517/2572-8180.2017.15. - DOI
-
- Steenbergen P.J., Bardine N. Antinociceptive effects of buprenorphine in zebrafish larvae: An alternative for rodent models to study pain and nociception? Appl. Anim. Behav. Sci. 2014;152:92–99. doi: 10.1016/j.applanim.2013.12.001. - DOI
LinkOut - more resources
Full Text Sources

