Meropenem Plasma and Interstitial Soft Tissue Concentrations in Obese and Nonobese Patients-A Controlled Clinical Trial
- PMID: 33371322
- PMCID: PMC7767385
- DOI: 10.3390/antibiotics9120931
Meropenem Plasma and Interstitial Soft Tissue Concentrations in Obese and Nonobese Patients-A Controlled Clinical Trial
Abstract
Background: This controlled clinical study aimed to investigate the impact of obesity on plasma and tissue pharmacokinetics of meropenem.
Methods: Obese (body mass index (BMI) ≥ 35 kg/m2) and age-/sex-matched nonobese (18.5 kg/m2 ≥ BMI ≤ 30 kg/m2) surgical patients received a short-term infusion of 1000-mg meropenem. Concentrations were determined via high performance liquid chromatography-ultraviolet (HPLC-UV) in the plasma and microdialysate from the interstitial fluid (ISF) of subcutaneous tissue up to eight h after dosing. An analysis was performed in the plasma and ISF by noncompartmental methods.
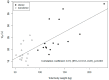
Results: The maximum plasma concentrations in 15 obese (BMI 49 ± 11 kg/m2) and 15 nonobese (BMI 24 ± 2 kg/m2) patients were 54.0 vs. 63.9 mg/L (95% CI for difference: -18.3 to -3.5). The volume of distribution was 22.4 vs. 17.6 L, (2.6-9.1), but the clearance was comparable (12.5 vs. 11.1 L/h, -1.4 to 3.1), leading to a longer half-life (1.52 vs. 1.31 h, 0.05-0.37) and fairly similar area under the curve (AUC)8h (78.7 vs. 89.2 mg*h/L, -21.4 to 8.6). In the ISF, the maximum concentrations differed significantly (12.6 vs. 18.6 L, -16.8 to -0.8) but not the AUC8h (28.5 vs. 42.0 mg*h/L, -33.9 to 5.4). Time above the MIC (T > MIC) in the plasma and ISF did not differ significantly for MICs of 0.25-8 mg/L.
Conclusions: In morbidly obese patients, meropenem has lower maximum concentrations and higher volumes of distribution. However, due to the slightly longer half-life, obesity has no influence on the T > MIC, so dose adjustments for obesity seem unnecessary.
Keywords: antibiotic dosing; concentrations; meropenem; microdialysis; obesity; pharmacodynamics; pharmacokinetics; soft tissue.
Conflict of interest statement
H.W. received grants from Pfizer (Investigator Initiated Trial Program, Berlin, Germany) and InfectoPharm (Heppenheim, Germany), both for the clinical microdialysis trial. H.W. reports lecture fees from InfectoPharm (Heppenheim, Germany), MSD (Konstanz, Germany) and consultant honoraria from Dräger Medical (Lübeck, Germany). P.S. reports lecture fees from InfectoPharm (Heppenheim, Germany). C.K. reports grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG, AstraZeneca GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Grünenthal GmbH, F. Hoffmann-La Roche Ltd., Merck KGaA and SANOFI) for the PharMetrX program, grants for the Innovative Medicines Initiative-Joint Undertaking (“DDMoRe”), Diurnal Ltd., the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (JPIAMR) and from the European Commission within in the Horizon 2020 framework programme (“FAIR”). M.Z. received funding for other investigator initiated trials from Pfizer (Wien, Austria). The other authors declare that they have no competing interests.
Figures


References
-
- Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., Kumar A., Sevransky J.E., Sprung C.L., Nunnally M.E., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. - DOI - PubMed
-
- Brinkmann A., Röhr A.C., Frey O.R., Krüger W.A., Brenner T., Richter D.C., Bodmann K.F., Kresken M., Grabein B. S2k guidelines of the PEG on calculated parenteral initial treatment of bacterial diseases in adults: Focussed summary and supplementary information on antibiotic treatment of critically ill patients. Anaesthesist. 2018;67:936–949. doi: 10.1007/s00101-018-0512-8. - DOI - PubMed
-
- World Health Organization Obesity and Overweight. [(accessed on 29 September 2009)]; Available online: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
Grants and funding
LinkOut - more resources
Full Text Sources

