Targeted Isolation of Anti-Trypanosomal Naphthofuran-Quinone Compounds from the Mangrove Plant Avicennia lanata
- PMID: 33371387
- PMCID: PMC7767399
- DOI: 10.3390/md18120661
Targeted Isolation of Anti-Trypanosomal Naphthofuran-Quinone Compounds from the Mangrove Plant Avicennia lanata
Abstract
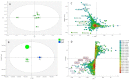
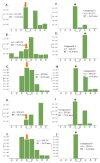
The discovery of new secondary metabolites from natural origins has become more challenging in natural products research. Different approaches have been applied to target the isolation of new bioactive metabolites from plant extracts. In this study, bioactive natural products were isolated from the crude organic extract of the mangrove plant Avicennia lanata collected from the east coast of Peninsular Malaysia in the Setiu Wetlands, Terengganu, using HRESI-LCMS-based metabolomics-guided isolation and fractionation. Isolation work on the crude extract A. lanata used high-throughput chromatographic techniques to give two new naphthofuranquinone derivatives, hydroxyavicenol C (1) and glycosemiquinone (2), along with the known compounds avicenol C (3), avicequinone C (4), glycoquinone (5), taraxerone (6), taraxerol (7), β-sitosterol (8) and stigmasterol (9). The elucidation and identification of the targeted bioactive compounds used 1D and 2D-NMR and mass spectrometry. Except for 6-9, all isolated naphthoquinone compounds (1-5) from the mangrove plant A. lanata showed significant anti-trypanosomal activity on Trypanosoma brucei brucei with MIC values of 3.12-12.5 μM. Preliminary cytotoxicity screening against normal prostate cells (PNT2A) was also performed. All compounds exhibited low cytotoxicity, with compounds 3 and 4 showing moderate cytotoxicity of 78.3% and 68.6% of the control values at 100 μg/mL, respectively.
Keywords: NMR; dereplication; high-throughput chromatographic; mangrove; mass spectrometry; metabolic profiling; multivariate analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bandaranayake W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002;10:421–452. doi: 10.1023/A:1021397624349. - DOI
-
- Duke N., Ball M., Ellison J. Factors influencing biodiversity and distributional gradients in mangroves. Global Ecol. Biogeogr. Lett. 1998;7:27–47. doi: 10.2307/2997695. - DOI
-
- Peter K.L.N., Sivasothi N. A Guide to the Mangroves of Singapore 1: The Ecosystem & Plant. Diversity. Singapore Science Centre; Singapore: 1999. revised edition (2002)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

