Breast Cancer Organoids Model Patient-Specific Response to Drug Treatment
- PMID: 33371412
- PMCID: PMC7770601
- DOI: 10.3390/cancers12123869
Breast Cancer Organoids Model Patient-Specific Response to Drug Treatment
Abstract
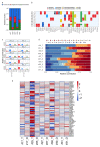
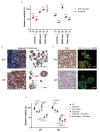
Tumor organoids are tridimensional cell culture systems that are generated in vitro from surgically resected patients' tumors. They can be propagated in culture maintaining several features of the tumor of origin, including cellular and genetic heterogeneity, thus representing a promising tool for precision cancer medicine. Here, we established patient-derived tumor organoids (PDOs) from different breast cancer subtypes (luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and triple negative). The established model systems showed histological and genomic concordance with parental tumors. However, in PDOs, the ratio of diverse cell populations was frequently different from that originally observed in parental tumors. We showed that tumor organoids represent a valuable system to test the efficacy of standard therapeutic treatments and to identify drug resistant populations within tumors. We also report that inhibitors of mechanosignaling and of Yes-associated protein 1 (YAP) activation can restore chemosensitivity in drug resistant tumor organoids.
Keywords: YAP; breast cancer; dasatinib; drug testing; heterogeneity; mechanotransduction; patient-derived tumor organoids; statin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Leight J.L., Drain A.P., Weaver V.M. Extracellular Matrix Remodeling and Stiffening Modulate Tumor Phenotype and Treatment Response. Annu. Rev. Cancer Biol. 2017;1:313–334. doi: 10.1146/annurev-cancerbio-050216-034431. - DOI
Grants and funding
- ITAT1096-P/European Union, European Regional Development Fund and Interreg V-A Italia-Austria 2014-2020
- PRIN-2017HWTP2K_004/Italian University and Research Ministry
- 22174/Associazione Italiana per la Ricerca sul Cancro
- 22759/Associazione Italiana per la Ricerca sul Cancro
- 10016/Associazione Italiana per la Ricerca sul Cancro
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

