Endothelial to Mesenchymal Transition in Pulmonary Vascular Diseases
- PMID: 33371458
- PMCID: PMC7767472
- DOI: 10.3390/biomedicines8120639
Endothelial to Mesenchymal Transition in Pulmonary Vascular Diseases
Abstract
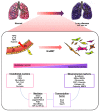
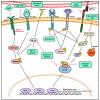
Lung diseases, such as pulmonary hypertension and pulmonary fibrosis, are life-threatening diseases and have common features of vascular remodeling. During progression, extracellular matrix protein deposition and dysregulation of proteolytic enzymes occurs, which results in vascular stiffness and dysfunction. Although vasodilators or anti-fibrotic therapy have been mainly used as therapy owing to these characteristics, their effectiveness does not meet expectations. Therefore, a better understanding of the etiology and new therapeutic approaches are needed. Endothelial cells (ECs) line the inner walls of blood vessels and maintain vascular homeostasis by protecting vascular cells from pathological stimuli. Chronic stimulation of ECs by various factors, including pro-inflammatory cytokines and hypoxia, leads to ECs undergoing an imbalance of endothelial homeostasis, which results in endothelial dysfunction and is closely associated with vascular diseases. Emerging studies suggest that endothelial to mesenchymal transition (EndMT) contributes to endothelial dysfunction and plays a key role in the pathogenesis of vascular diseases. EndMT is a process by which ECs lose their markers and show mesenchymal-like morphological changes, and gain mesenchymal cell markers. Despite the efforts to elucidate these molecular mechanisms, the role of EndMT in the pathogenesis of lung disease still requires further investigation. Here, we review the importance of EndMT in the pathogenesis of pulmonary vascular diseases and discuss various signaling pathways and mediators involved in the EndMT process. Furthermore, we will provide insight into the therapeutic potential of targeting EndMT.
Keywords: endothelial to mesenchymal transition; lung disease; pulmonary fibrosis; pulmonary hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

