Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins
- PMID: 33371477
- PMCID: PMC7767516
- DOI: 10.3390/toxins12120812
Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins
Abstract
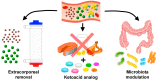
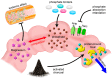
Uremic vascular calcification (VC) commonly occurs during advanced chronic kidney disease (CKD) and significantly increases cardiovascular morbidity and mortality. Uremic toxins are integral within VC pathogenesis, as they exhibit adverse vascular influences ranging from atherosclerosis, vascular inflammation, to VC. Experimental removal of these toxins, including small molecular (phosphate, trimethylamine-N-oxide), large molecular (fibroblast growth factor-23, cytokines), and protein-bound ones (indoxyl sulfate, p-cresyl sulfate), ameliorates VC. As most uremic toxins share a gut origin, interventions through gastrointestinal tract are expected to demonstrate particular efficacy. The "gastrointestinal decontamination" through the removal of toxin in situ or impediment of toxin absorption within the gastrointestinal tract is a practical and potential strategy to reduce uremic toxins. First and foremost, the modulation of gut microbiota through optimizing dietary composition, the use of prebiotics or probiotics, can be implemented. Other promising strategies such as reducing calcium load, minimizing intestinal phosphate absorption through the optimization of phosphate binders and the inhibition of gut luminal phosphate transporters, the administration of magnesium, and the use of oral toxin adsorbent for protein-bound uremic toxins may potentially counteract uremic VC. Novel agents such as tenapanor have been actively tested in clinical trials for their potential vascular benefits. Further advanced studies are still warranted to validate the beneficial effects of gastrointestinal decontamination in the retardation and treatment of uremic VC.
Keywords: aortic calcification; chronic kidney disease; chronic kidney disease-mineral bone disorder; indoxyl sulfate; oral adsorbent; uremic toxin; vascular calcification; vascular smooth muscle cell.
Conflict of interest statement
The authors have no relevant financial or non-financial competing interests to declare in relation to this manuscript.
Figures

References
-
- Bundy J.D., Cai X., Scialla J.J., Dobre M.A., Chen J., Hsu C.-Y., Leonard M.B., Go A.S., Rao P.S., Lash J.P., et al. Serum calcification propensity and coronary artery calcification among patients with CKD: The CRIC (Chronic Renal Insufficiency Cohort) study. Am. J. Kidney Dis. 2019;73:806–814. doi: 10.1053/j.ajkd.2019.01.024. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

