Nuclear Medicine in Times of COVID-19: How Radiopharmaceuticals Could Help to Fight the Current and Future Pandemics
- PMID: 33371500
- PMCID: PMC7767508
- DOI: 10.3390/pharmaceutics12121247
Nuclear Medicine in Times of COVID-19: How Radiopharmaceuticals Could Help to Fight the Current and Future Pandemics
Abstract
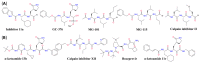
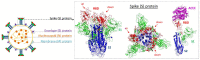
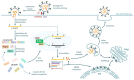
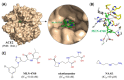
The emergence and global spread of COVID-19, an infectious disease caused by the novel coronavirus SARS-CoV-2, has resulted in a continuing pandemic threat to global health. Nuclear medicine techniques can be used for functional imaging of (patho)physiological processes at the cellular or molecular level and for treatment approaches based on targeted delivery of therapeutic radionuclides. Ongoing development of radiolabeling methods has significantly improved the accessibility of radiopharmaceuticals for in vivo molecular imaging or targeted radionuclide therapy, but their use for biosafety threats such as SARS-CoV-2 is restricted by the contagious nature of these agents. Here, we highlight several potential uses of nuclear medicine in the context of SARS-CoV-2 and COVID-19, many of which could also be performed in laboratories without dedicated containment measures. In addition, we provide a broad overview of experimental or repurposed SARS-CoV-2-targeting drugs and describe how radiolabeled analogs of these compounds could facilitate antiviral drug development and translation to the clinic, reduce the incidence of late-stage failures and possibly provide the basis for radionuclide-based treatment strategies. Based on the continuing threat by emerging coronaviruses and other pathogens, it is anticipated that these applications of nuclear medicine will become a more important part of future antiviral drug development and treatment.
Keywords: PET-based antiviral drug development; diagnostic radionuclides; molecular imaging of SARS-CoV-2; positron emission tomography (PET); radioimmunotherapy (RIT); radionuclide therapy of COVID-19; single photon emission computer tomography (SPECT); therapeutic radionuclides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Current Strategies of Antiviral Drug Discovery for COVID-19.Front Mol Biosci. 2021 May 13;8:671263. doi: 10.3389/fmolb.2021.671263. eCollection 2021. Front Mol Biosci. 2021. PMID: 34055887 Free PMC article. Review.
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
-
Potential Utility of Radiopharmaceuticals in the Battle Against SARSCov- 2 and COVID-19 Pandemic.Curr Radiopharm. 2022;15(2):93-95. doi: 10.2174/1874471014666211011122250. Curr Radiopharm. 2022. PMID: 34635047
-
Diagnostic, Prognostic, and Therapeutic Use of Radiopharmaceuticals in the Context of SARS-CoV-2.ACS Pharmacol Transl Sci. 2021 Jan 15;4(1):1-7. doi: 10.1021/acsptsci.0c00186. eCollection 2021 Feb 12. ACS Pharmacol Transl Sci. 2021. PMID: 33615159 Free PMC article. Review.
-
Positron emission tomography for the assessment of myocardial viability: an evidence-based analysis.Ont Health Technol Assess Ser. 2010;10(16):1-80. Epub 2010 Jul 1. Ont Health Technol Assess Ser. 2010. PMID: 23074393 Free PMC article.
Cited by
-
Nuclear Medicine During the COVID-19 Pandemic: The Show Must Go On.Front Med (Lausanne). 2022 May 12;9:896069. doi: 10.3389/fmed.2022.896069. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35646988 Free PMC article. No abstract available.
-
Mutated Isocitrate Dehydrogenase (mIDH) as Target for PET Imaging in Gliomas.Molecules. 2023 Mar 23;28(7):2890. doi: 10.3390/molecules28072890. Molecules. 2023. PMID: 37049661 Free PMC article. Review.
-
The Impact of COVID-19 on Nuclear Medicine Operations Including Cardiovascular Manifestations in the USA.Semin Nucl Med. 2022 Jan;52(1):11-16. doi: 10.1053/j.semnuclmed.2021.06.003. Epub 2021 Jun 17. Semin Nucl Med. 2022. PMID: 34246451 Free PMC article. Review.
-
Synthesis and Inhibitory Assessment of ACE2 Inhibitors for SARS-CoV-2: An In Silico and In Vitro Study.J Org Chem. 2025 Aug 1;90(30):10941-10947. doi: 10.1021/acs.joc.5c00918. Epub 2025 Jul 21. J Org Chem. 2025. PMID: 40686086 Free PMC article.
-
Noninvasive Imaging for Patients with COVID-19 and Acute Chest Pain.Methodist Debakey Cardiovasc J. 2021 Dec 15;17(5):5-15. doi: 10.14797/mdcvj.1040. eCollection 2021. Methodist Debakey Cardiovasc J. 2021. PMID: 34992719 Free PMC article. Review.
References
-
- Davis S.L., Nuermberger E.L., Um P.K., Vidal C., Jedynak B., Pomper M.G., Bishai W.R., Jain S.K. Noninvasive Pulmonary [18F]-2-Fluoro-Deoxy-d-Glucose Positron Emission Tomography Correlates with Bactericidal Activity of Tuberculosis Drug Treatment. Antimicrob. Agents Chemother. 2009;53:4879–4884. doi: 10.1128/AAC.00789-09. - DOI - PMC - PubMed
-
- Jahrling P.B., Keith L., Claire M.S., Johnson R.F., Bollinger L., Lackemeyer M.G., Hensley L.E., Kindrachuk J., Kuhn J.H. The NIAID Integrated Research Facility at Frederick, Maryland: A unique international resource to facilitate medical countermeasure development for BSL-4 pathogens. Pathog. Dis. 2014;71:213–219. doi: 10.1111/2049-632X.12171. - DOI - PMC - PubMed
-
- Hartman A.L., Nambulli S., McMillen C.M., White A.G., Tilston-Lunel N.L., Albe J.R., Cottle E., Dunn M.D., Frye L.J., Gilliland T.H., et al. SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLoS Pathog. 2020;16:e1008903. doi: 10.1371/journal.ppat.1008903. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

