Stopping Renin-Angiotensin System Inhibitors in Patients with Advanced CKD and Risk of Adverse Outcomes: A Nationwide Study
- PMID: 33372009
- PMCID: PMC8054897
- DOI: 10.1681/ASN.2020050682
Stopping Renin-Angiotensin System Inhibitors in Patients with Advanced CKD and Risk of Adverse Outcomes: A Nationwide Study
Abstract
Background: It is unknown whether stopping renin-angiotensin system (RAS) inhibitor therapy in patients with advanced CKD affects outcomes.
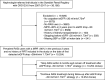
Methods: We studied patients referred to nephrologist care, listed on the Swedish Renal Registry during 2007-2017, who developed advanced CKD (eGFR<30 ml/min per 1.73 m2) while on RAS inhibitor therapy. Using target trial emulation techniques on the basis of cloning, censoring, and weighting, we compared the risks of stopping within 6 months and remaining off treatment versus continuing RAS inhibitor therapy. These included risks of subsequent 5-year all-cause mortality, major adverse cardiovascular events, and initiation of kidney replacement therapy (KRT).
Results: Of 10,254 prevalent RAS inhibitor users (median age 72 years, 36% female) with new-onset eGFR <30 ml/min per 1.73 m2, 1553 (15%) stopped RAS inhibitor therapy within 6 months. Median eGFR was 23 ml/min per 1.73 m2. Compared with continuing RAS inhibition, stopping this therapy was associated with a higher absolute 5-year risk of death (40.9% versus 54.5%) and major adverse cardiovascular events (47.6% versus 59.5%), but with a lower risk of KRT (36.1% versus 27.9%); these corresponded to absolute risk differences of 13.6 events per 100 patients, 11.9 events per 100 patients, and -8.3 events per 100 patients, respectively. Results were consistent whether patients stopped RAS inhibition at higher or lower eGFR, across prespecified subgroups, after adjustment and stratification for albuminuria and potassium, and when modeling RAS inhibition as a time-dependent exposure using a marginal structural model.
Conclusions: In this nationwide observational study of people with advanced CKD, stopping RAS inhibition was associated with higher absolute risks of mortality and major adverse cardiovascular events, but also with a lower absolute risk of initiating KRT.
Keywords: ACE inhibitors; cardiovascular events; dialysis; kidney disease; mortality risk; renin-angiotensin system.
Copyright © 2021 by the American Society of Nephrology.
Figures


Similar articles
-
Comparative Effectiveness of Renin-Angiotensin System Inhibitors and Calcium Channel Blockers in Individuals With Advanced CKD: A Nationwide Observational Cohort Study.Am J Kidney Dis. 2021 May;77(5):719-729.e1. doi: 10.1053/j.ajkd.2020.10.006. Epub 2020 Nov 24. Am J Kidney Dis. 2021. PMID: 33246024
-
Detrimental effect of renin-angiotensin blockade on progression of chronic kidney disease at later stages: A matter of dosage adjustment?Nefrologia (Engl Ed). 2020 Jan-Feb;40(1):38-45. doi: 10.1016/j.nefro.2019.02.013. Epub 2019 Jun 10. Nefrologia (Engl Ed). 2020. PMID: 31196659 English, Spanish.
-
Effects of Stenting for Atherosclerotic Renal Artery Stenosis on eGFR and Predictors of Clinical Events in the CORAL Trial.Clin J Am Soc Nephrol. 2016 Jul 7;11(7):1180-1188. doi: 10.2215/CJN.10491015. Epub 2016 May 25. Clin J Am Soc Nephrol. 2016. PMID: 27225988 Free PMC article. Clinical Trial.
-
Use of Renin-Angiotensin System Blockade in Advanced CKD: An NKF-KDOQI Controversies Report.Am J Kidney Dis. 2018 Dec;72(6):873-884. doi: 10.1053/j.ajkd.2018.06.010. Epub 2018 Sep 7. Am J Kidney Dis. 2018. PMID: 30201547 Review.
-
A systematic review and meta-analysis of the clinical impact of stopping renin-angiotensin system inhibitor in patients with chronic kidney disease.Hypertens Res. 2023 Jun;46(6):1525-1535. doi: 10.1038/s41440-023-01260-8. Epub 2023 Mar 28. Hypertens Res. 2023. PMID: 36977900
Cited by
-
Care of Adults with Advanced Chronic Kidney Disease.J Clin Med. 2024 Jul 26;13(15):4378. doi: 10.3390/jcm13154378. J Clin Med. 2024. PMID: 39124645 Free PMC article. Review.
-
Clinical outcomes following discontinuation of renin-angiotensin-system inhibitors in patients with type 2 diabetes and advanced chronic kidney disease: A prospective cohort study.EClinicalMedicine. 2022 Nov 24;55:101751. doi: 10.1016/j.eclinm.2022.101751. eCollection 2023 Jan. EClinicalMedicine. 2022. PMID: 36457651 Free PMC article.
-
The effect of kidney function on guideline-directed medical therapy implementation and prognosis in heart failure with reduced ejection fraction.Clin Cardiol. 2024 Feb;47(2):e24244. doi: 10.1002/clc.24244. Clin Cardiol. 2024. PMID: 38402552 Free PMC article.
-
Dose and Time Effects of Renin-Angiotensin Inhibitors on Patients With Advanced Stages 4 to 5 of Diabetic Kidney Disease.J Endocr Soc. 2024 Jun 11;8(8):bvae119. doi: 10.1210/jendso/bvae119. eCollection 2024 Jul 1. J Endocr Soc. 2024. PMID: 38979403 Free PMC article.
-
Treatment of chronic kidney disease in older populations.Nat Rev Nephrol. 2024 Sep;20(9):586-602. doi: 10.1038/s41581-024-00854-w. Epub 2024 Jul 8. Nat Rev Nephrol. 2024. PMID: 38977884 Review.
References
-
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. .; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 - PubMed
-
- Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, et al. .: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 135: 73–87, 2001 - PubMed
-
- Kent DM, Jafar TH, Hayward RA, Tighiouart H, Landa M, de Jong P, et al. .: Progression risk, urinary protein excretion, and treatment effects of angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol 18: 1959–1965, 2007 - PubMed
-
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. .; Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

