Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS
- PMID: 33372052
- PMCID: PMC8592388
- DOI: 10.1136/jnnp-2020-325121
Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS
Abstract
Objective: To determine the frequency and characteristics of brainstem or cerebellar involvement in myelin-oligodendrocyte-glycoprotein-antibody-associated-disorder (MOGAD) versus aquaporin-4-IgG-seropositive-neuromyelitis optica spectrum disorder (AQP4-IgG-NMOSD) and multiple sclerosis (MS).
Methods: In this observational study, we retrospectively identified 185 Mayo Clinic MOGAD patients with: (1) characteristic MOGAD phenotype, (2) MOG-IgG seropositivity by live cell-based assay and (3) MRI lesion(s) of brainstem, cerebellum or both. We compared the symptomatic attacks to AQP4-IgG-NMOSD (n=30) and MS (n=30).
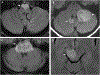
Results: Brainstem or cerebellar involvement occurred in 62/185 (34%) MOGAD patients of which 39/62 (63%) were symptomatic. Ataxia (45%) and diplopia (26%) were common manifestations. The median age in years (range) in MOGAD of 24 (2-65) was younger than MS at 36 (16-65; p=0.046) and AQP4-IgG-NMOSD at 45 (6-72; p=0.006). Isolated attacks involving the brainstem, cerebellum or both were less frequent in MOGAD (9/39 (23%)) than MS (22/30 (73%); p<0.001) but not significantly different from AQP4-IgG-NMOSD (14/30 (47%); p=0.07). Diffuse middle cerebellar peduncle MRI-lesions favoured MOGAD (17/37 (46%)) over MS (3/30 (10%); p=0.001) and AQP4-IgG-NMOSD (3/30 (10%); p=0.001). Diffuse medulla, pons or midbrain MRI lesions occasionally occurred in MOGAD and AQP4-IgG-NMOSD but never in MS. Cerebrospinal fluid (CSF) oligoclonal bands were rare in MOGAD (5/30 (17%)) and AQP4-IgG-NMOSD (2/22 (9%); p=0.68) but common in MS (18/22 (82%); p<0.001). Disability at nadir or recovery did not differ between the groups.
Conclusion: Involvement of the brainstem, cerebellum or both is common in MOGAD but usually occurs as a component of a multifocal central nervous system attack rather than in isolation. We identified clinical, CSF and MRI attributes that can help discriminate MOGAD from AQP4-IgG-NMOSD and MS.
© Author(s) (or their employer(s)) 2020. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SAB: Reports no disclosures PPM: Reports no disclosures. JJC: Reports no disclosures. SJP: Reports grants, personal fees and non-financial support from Alexion Pharmaceuticals; grants from Grifols, Autoimmune Encephalitis Alliance; grants, personal fees, non-financial support and other from MedImmune; SJP has a patent # 9,891,219 (Application#12-573942) 'Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an individual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive'. SJP also has patents pending for the following IgGs as biomarkers of autoimmune neurological disorders (septin-5, Kelch-like protein 11, GFAP, PDE10A and MAP1B ES: Reports no disclosures. AK: Reports no disclosures J-MT: Reports no disclosures. JPF: Reports no disclosures. BGW: Receives royalties from RSR, Oxford University, Hospices Civil de Lyon, and MVZ Labor PD Dr Volkmann und Kollegen GbR for a patent of NMO-IgG as a diagnostic test for neuromyelitis optica spectrum disorders, served on adjudication committee for clinical trials in neuromyelitis optica spectrum disorders being conducted by MedImmune/VielaBio and Alexion, and consulted for Chugai/Roche/Genentech and Mitsubishi-Tanabe regarding clinical trials for neuromyelitis optica spectrum disorders. KNK: Reports no disclosures ASL-C: Reports no disclosures. AN: Reports no disclosures TMG: Reports no disclosures. CL: Reports no disclosures SAM: Reports no disclosures. EPF: EPF is a site principal investigator in a randomiSed placebo-controlled clinical trial of Inebilizumab (A CD19 inhibitor) in neuromyelitis optica spectrum disorders funded by MedImmune/Viela Bio.
Figures


References
-
- Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nature Reviews Neurology. 2019;15(2):89–102. - PubMed
-
- Jurynczyk M, Geraldes R, Probert F, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. 2017;140(3):617–627. - PubMed
-
- Baumann M, Grams A, Djurdjevic T, et al. MRI of the first event in pediatric acquired demyelinating syndromes with antibodies to myelin oligodendrocyte glycoprotein. Journal of neurology. 2018;265(4):845–855. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
