Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial)
- PMID: 33372103
- PMCID: PMC7783616
- DOI: 10.1136/bmjgast-2020-000531
Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial)
Abstract
Introduction: Cirrhotic patients with portal hypertension can suffer from variceal bleeding or refractory ascites and can benefit from a transjugular intrahepatic portosystemic shunt (TIPS). Post-TIPS hepatic encephalopathy (HE) is a common (20%-54%) and often severe complication. A prophylactic strategy is lacking.
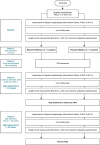
Methods and analysis: The Prevention of hepatic Encephalopathy by Administration of Rifaximin and Lactulose in patients with liver cirrhosis undergoing placement of a TIPS (PEARL) trial, is a multicentre randomised, double blind, placebo controlled trial. Patients undergoing covered TIPS placement are prescribed either rifaximin 550 mg two times per day and lactulose 25 mL two times per day (starting dose) or placebo 550 mg two times per day and lactulose 25 mL two times per day from 72 hours before and until 3 months after TIPS placement. Primary endpoint is the development of overt HE (OHE) within 3 months (according to West Haven criteria). Secondary endpoints include 90-day mortality; development of a second episode of OHE; time to development of episode(s) of OHE; development of minimal HE; molecular changes in peripheral and portal blood samples; quality of life and cost-effectiveness. The total sample size is 238 patients and recruitment period is 3 years in six hospitals in the Netherlands and one in Belgium.
Ethics and dissemination: This study protocol was approved in the Netherlands by the Medical Research Ethics Committee of the Academic Medical Centre, Amsterdam (2018-332), in Belgium by the Ethics Committee Research UZ/KU Leuven (S62577) and competent authorities. This study will be conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Study results will be submitted for publication in a peer-reviewed journal.
Trial registration numbers: ClinicalTrials.gov (NCT04073290) and EudraCT database (2018-004323-37).
Keywords: hepatic encephalopathy; liver cirrhosis; portal hypertension.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MK: lecture fees from Norgine. BT: grant from Netherlands Organisation for Health Research and Development; advisory board member of Norgine. UB: grants from Dr Falk Pharma and Intercept for investigator-initiated studies; lecture fees from Abbvie, Falk Foundation, Gilead, Intercept.
Figures
References
-
- Runyon BA. Management of adult patients with ascites due to cirrhosis: update, 2012. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical