Affinity of small-molecule solutes to hydrophobic, hydrophilic, and chemically patterned interfaces in aqueous solution
- PMID: 33372161
- PMCID: PMC7821046
- DOI: 10.1073/pnas.2020205118
Affinity of small-molecule solutes to hydrophobic, hydrophilic, and chemically patterned interfaces in aqueous solution
Abstract
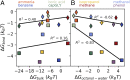
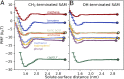
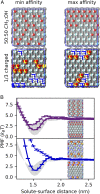
Performance of membranes for water purification is highly influenced by the interactions of solvated species with membrane surfaces, including surface adsorption of solutes upon fouling. Current efforts toward fouling-resistant membranes often pursue surface hydrophilization, frequently motivated by macroscopic measures of hydrophilicity, because hydrophobicity is thought to increase solute-surface affinity. While this heuristic has driven diverse membrane functionalization strategies, here we build on advances in the theory of hydrophobicity to critically examine the relevance of macroscopic characterizations of solute-surface affinity. Specifically, we use molecular simulations to quantify the affinities to model hydroxyl- and methyl-functionalized surfaces of small, chemically diverse, charge-neutral solutes represented in produced water. We show that surface affinities correlate poorly with two conventional measures of solute hydrophobicity, gas-phase water solubility and oil-water partitioning. Moreover, we find that all solutes show attraction to the hydrophobic surface and most to the hydrophilic one, in contrast to macroscopically based hydrophobicity heuristics. We explain these results by decomposing affinities into direct solute interaction energies (which dominate on hydroxyl surfaces) and water restructuring penalties (which dominate on methyl surfaces). Finally, we use an inverse design algorithm to show how heterogeneous surfaces, with multiple functional groups, can be patterned to manipulate solute affinity and selectivity. These findings, importantly based on a range of solute and surface chemistries, illustrate that conventional macroscopic hydrophobicity metrics can fail to predict solute-surface affinity, and that molecular-scale surface chemical patterning significantly influences affinity-suggesting design opportunities for water purification membranes and other engineered interfaces involving aqueous solute-surface interactions.
Keywords: inverse design; membrane fouling; molecular simulation; solvation free energy; surface adsorption.
Conflict of interest statement
The authors declare no competing interest.
Figures

Comment in
-
Navigating the waters of membrane design.Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2024346118. doi: 10.1073/pnas.2024346118. Proc Natl Acad Sci U S A. 2021. PMID: 33384327 Free PMC article. No abstract available.
References
-
- Arai K., Bhatia R., Kapoor S., Eds. Proceedings of the Future Technologies Conference (FTC), Vol. 1 (Springer Nature, 2019).
-
- Werber J. R., Osuji C. O., Elimelech M., Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 16018 (2016).
-
- Park H. B., Kamcev J., Robeson L. M., Elimelech M., Freeman B. D., Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017). - PubMed
-
- Fritzmann C., Löwenberg J., Wintgens T., Melin T., State-of-the-art of reverse osmosis desalination. Desalination 216, 1–76 (2007).
-
- Miller D. J., Dreyer D. R., Bielawski C. W., Paul D. R., Freeman B. D., Surface modification of water purification membranes. Angew. Chem. Int. Ed. Engl. 56, 4662–4711 (2017). - PubMed

