Leptomeningeal metastatic cells adopt two phenotypic states
- PMID: 33372403
- PMCID: PMC7772527
- DOI: 10.1002/cnr2.1236
Leptomeningeal metastatic cells adopt two phenotypic states
Abstract
Background: Leptomeningeal metastasis (LM), or spread of cancer cells into the cerebrospinal fluid (CSF), is characterized by a rapid onset of debilitating neurological symptoms and markedly bleak prognosis. The lack of reproducible in vitro and in vivo models has prevented the development of novel, LM-specific therapies. Although LM allows for longitudinal sampling of floating cancer cells with a spinal tap, attempts to culture patient-derived leptomeningeal cancer cells have not been successful.
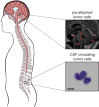
Aim: We, therefore, employ leptomeningeal derivatives of human breast and lung cancer cell lines that reproduce both floating and adherent phenotypes of human LM in vivo and in vitro.
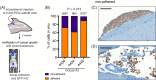
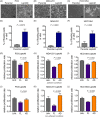
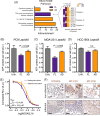
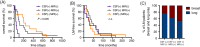
Methods and results: We introduce a trypsin/EDTA-based fractionation method to reliably separate the two cell subsets and demonstrate that in vitro cultured floating cells have decreased proliferation rate, lower ATP content, and are enriched in distinct metabolic signatures. Long-term fractionation and transcriptomic analysis suggest high degree plasticity between the two phenotypes in vitro. Floating cells colonize mouse leptomeninges more rapidly and associate with shortened survival. In addition, patients harboring LM diagnosed with CSF disease alone succumbed to the disease earlier than patients with adherent (MRI positive) disease.
Conclusion: Together, these data support mechanistic evidence of a metabolic adaptation that allows cancer cells to thrive in their natural environment but leads to death in vitro.
Keywords: cancer plasticity; cerebrospinal fluid; electron transport chain; leptomeningeal metastasis; metabolic adaptation.
© 2020 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
Adrienne Boire has consulted for Arix Bioscience (2018), is on the Scientific Advisory Board for Evren Scientific (unpaid), and holds patent applications: 62/258044 and 62/052966. Other authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

