Tumor infiltrating neutrophils and gland formation predict overall survival and molecular subgroups in pancreatic ductal adenocarcinoma
- PMID: 33372414
- PMCID: PMC7897949
- DOI: 10.1002/cam4.3695
Tumor infiltrating neutrophils and gland formation predict overall survival and molecular subgroups in pancreatic ductal adenocarcinoma
Abstract
Background: RNA-sequencing-based classifiers can stratify pancreatic ductal adenocarcinoma (PDAC) into prognostically significant subgroups but are not practical for use in clinical workflows. Here, we assess whether histomorphological features may be used as surrogate markers for predicting molecular subgroup and overall survival in PDAC.
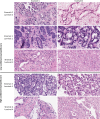
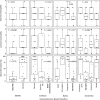
Methods: Ninety-six tissue samples from 50 patients with non-resectable PDAC were scored for gland formation, stromal maturity, mucin, necrosis, and neutrophil infiltration. Prognostic PDAC gene expression classifiers were run on all tumors using whole transcriptome sequencing data from the POG trial (NCT02155621). Findings were validated using digital TCGA slides (n = 50). Survival analysis used multivariate Cox proportional-hazards tests and log-rank tests.
Results: The combination of low gland formation and low neutrophil infiltration was significantly associated with the poor prognosis PDAC molecular subgroup (basal-like or squamous) and was an independent predictor of shorter overall survival, in both frozen section (n = 47) and formalin-fixed paraffin-embedded (n = 49) tissue samples from POG patients, and in the TCGA samples. This finding held true in the subgroup analysis of primary (n = 17) and metastatic samples (n = 79). The combination of high gland formation and high neutrophils had low sensitivity but high specificity for favorable prognosis subgroups.
Conclusions: The assessment of gland formation and neutrophil infiltration on routine histological sections can aid in prognostication and allow inferences to be made about molecular subtype, which may help guide patient management decisions and contribute to our understanding of heterogeneity in treatment response.
Keywords: molecular; pancreatic neoplasms; pathology; prognosis.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
D.F.S. reports consultant fees from Robarts Clinical Trials Inc, unrelated to the work presented. D.J.R. disclosures include research funding and honoraria from Bayer, and honoraria from Servier, Celgene, Taiho, and Ipsen. J.L. declares honoraria for academic talks from Roche Canada, BI Canada, AstraZeneca Canada, and research grants from Roche Canada, Pfizer Canada, and AstraZeneca Canada. The remaining authors have no conflicts of interests to declare.
Figures




Similar articles
-
Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma.Br J Surg. 2016 Aug;103(9):1189-99. doi: 10.1002/bjs.10187. Epub 2016 Jun 3. Br J Surg. 2016. PMID: 27256393
-
A PD-L2-based immune marker signature helps to predict survival in resected pancreatic ductal adenocarcinoma.J Immunother Cancer. 2019 Aug 29;7(1):233. doi: 10.1186/s40425-019-0703-0. J Immunother Cancer. 2019. PMID: 31464648 Free PMC article.
-
Time to CA19-9 nadir: a clue for defining optimal treatment duration in patients with resectable pancreatic ductal adenocarcinoma.Cancer Chemother Pharmacol. 2020 Apr;85(4):641-650. doi: 10.1007/s00280-020-04047-7. Epub 2020 Mar 10. Cancer Chemother Pharmacol. 2020. PMID: 32157412
-
Neutrophil in the Pancreatic Tumor Microenvironment.Biomolecules. 2021 Aug 7;11(8):1170. doi: 10.3390/biom11081170. Biomolecules. 2021. PMID: 34439836 Free PMC article. Review.
-
Novel Therapeutics for Pancreatic Adenocarcinoma.Hematol Oncol Clin North Am. 2015 Aug;29(4):777-87. doi: 10.1016/j.hoc.2015.04.006. Hematol Oncol Clin North Am. 2015. PMID: 26226910 Review.
Cited by
-
Advancing the Care of Pancreatic Cancer Patients: Moving Beyond Just Tumour Tissue.Biomark Insights. 2021 Oct 11;16:11772719211049852. doi: 10.1177/11772719211049852. eCollection 2021. Biomark Insights. 2021. PMID: 34658620 Free PMC article. Review.
-
Direct Endoplasmic Reticulum Targeting by the Selective Alkylphospholipid Analog and Antitumor Ether Lipid Edelfosine as a Therapeutic Approach in Pancreatic Cancer.Cancers (Basel). 2021 Aug 19;13(16):4173. doi: 10.3390/cancers13164173. Cancers (Basel). 2021. PMID: 34439330 Free PMC article. Review.
-
Integrated genomic analysis defines molecular subgroups in dilated cardiomyopathy and identifies novel biomarkers based on machine learning methods.Front Genet. 2023 Feb 7;14:1050696. doi: 10.3389/fgene.2023.1050696. eCollection 2023. Front Genet. 2023. PMID: 36824437 Free PMC article.
-
Immature stroma and high infiltration of CD15+ cells are predictive markers of poor prognosis in different subsets of patients with pancreatic cancer.Cancer Sci. 2024 Mar;115(3):1001-1013. doi: 10.1111/cas.16060. Epub 2024 Jan 17. Cancer Sci. 2024. PMID: 38230840 Free PMC article.
-
Heterogeneity of Glycan Biomarker Clusters as an Indicator of Recurrence in Pancreatic Cancer.bioRxiv [Preprint]. 2023 Jan 6:2023.01.05.522607. doi: 10.1101/2023.01.05.522607. bioRxiv. 2023. Update in: Front Oncol. 2023 Apr 12;13:1135405. doi: 10.3389/fonc.2023.1135405. PMID: 36711795 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

