French recommendations for the management of systemic necrotizing vasculitides (polyarteritis nodosa and ANCA-associated vasculitides)
- PMID: 33372616
- PMCID: PMC7771069
- DOI: 10.1186/s13023-020-01621-3
French recommendations for the management of systemic necrotizing vasculitides (polyarteritis nodosa and ANCA-associated vasculitides)
Erratum in
-
Correction to: French recommendations for the management of systemic necrotizing vasculitides (polyarteritis nodosa and ANCA-associated vasculitides).Orphanet J Rare Dis. 2021 Apr 6;16(1):155. doi: 10.1186/s13023-021-01787-4. Orphanet J Rare Dis. 2021. PMID: 33823903 Free PMC article. No abstract available.
Abstract
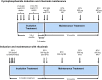
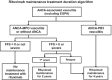
Systemic necrotizing vasculitis comprises a group of diseases resembling polyarteritis nodosa and anti-neutrophil cytoplasmic antibody-associated vasculitis (ANCA): granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and microscopic polyangiitis. The definitive diagnosis is made in cooperation with a reference center for autoimmune diseases and rare systemic diseases or a competency center. The management goals are: to obtain remission and, in the long term, healing; to reduce the risk of relapses; to limit and reduce the sequelae linked to the disease; to limit the side effects and the sequelae linked to the treatments; to improve or at least maintain the best possible quality of life; and to maintain socio-professional integration and/or allow a rapid return to school and/or professional activity. Information and therapeutic education of the patients and those around them are an integral part of the care. All health professionals and patients should be informed of the existence of patient associations. The treatment of vasculitis is based on variable combinations of glucocorticoids and immunosuppressants, chosen and adapted according to the disease concerned, the severity and/or extent of the disease, and the underlying factors (age, kidney function, etc.). Follow-up clinical and paraclinical examinations must be carried out regularly to clarify the progression of the disease, detect and manage treatment failures and possible relapses early on, and limit sequelae and complications (early then late) related to the disease or treatment. A distinction is made between the induction therapy, lasting approximately 3-6 months and aimed at putting the disease into remission, and the maintenance treatment, lasting 12-48 months, or even longer. The role of the increase or testing positive again for ANCA as a predictor of a relapse, which has long been controversial, now seems to have greater consensus: Anti-myeloperoxidase ANCAs are less often associated with a relapse of vasculitis than anti-PR3 ANCA.
Conflict of interest statement
BT reports consulting fees from Roche, AstraZeneca, and Roche-Chugai; speaking fees from Grifols. RD reports no conflicts of interest. CD reports no conflicts of interest. EH reports consulting fees from Actelion, Boehringer Ingelheim, Bayer, GSK, Roche-Chugai, Sanofi-Genzyme; speaking fees from Actelion, GSK, Roche-Chugai; and research funding from Octapharma, CSL Behring, GSK, Roche-Chugai, and Actelion. AK reports speaking fees from Roche. HM reports consulting fees from Amicus Therapeutics, Shire; speaking fees from Sanofi-Genzyme, Amicus Therapeutics. TP reports speaking fees from GSK, Ferring. XP has been an investigator in academic studies for which rituximab was provided by Roche Pharma; has declared speaking fees and honoraria (Boehringer Ingelheim, Sanofi, Pfizer, LFB), and congress inscription/travel/accommodation (Sanofi). GP reports no conflicts of interest. TQ reports research funding from Chemocentryx. MS reports consulting fees from Roche-Chugai; speaking fees from Roche-Chugai. CT reports consulting fees from Sanofi, GSK, Novartis, AstraZeneca; speaking fees from GSK, Sanofi, AstraZeneca; and research funding from GSK. LG reports consulting fees from Janssen, Lilly Pharma, AstraZeneca.
Figures
References
-
- Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol. 2004;93(4):398–401. - PubMed
-
- Bligny D, Mahr A, Toumelin PL, Mouthon L, Guillevin L. Predicting mortality in systemic Wegener’s granulomatosis: a survival analysis based on 93 patients. Arthritis Rheum. 2004;51(1):83–91. - PubMed
-
- Bloch DA, Michel BA, Hunder GG, McShane DJ, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Patients and methods. Arthritis Rheum. 1990;33(8):1068–1073. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials