Biosynthesis of the Immunosuppressant (-)-FR901483
- PMID: 33372776
- PMCID: PMC8094545
- DOI: 10.1021/jacs.0c12352
Biosynthesis of the Immunosuppressant (-)-FR901483
Abstract
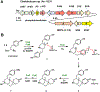
We report characterization of the biosynthetic pathway of the potent immunosuppressant (-)-FR901483 (1) through heterologous expression and enzymatic assays. The biosynthetic logic to form the azatricyclic alkaloid is consistent with those proposed in biomimetic syntheses and involves aza-spiro annulation of dityrosyl-piperazine to form a ketoaldehyde intermediate, followed by regioselective aldol condensation, stereoselective ketoreduction, and phosphorylation. A possible target of 1 is proposed based on the biosynthetic studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sakamoto K; Tsujii E; Abe F; Nakanishi T; Yamashita M; Shigematsu N; Izumi S; Okuhara M Fukuda T FR901483, a novel immunosuppressant isolated from Cladobotryum sp. No. 11231. Taxonomy of the producing organism, fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot 1996, 49, 37–44. - PubMed
-
- Ruan ZW; Li C; Shen DF; Huang S; Hong R FR901483: Synthetic efficiency remains a challenge. Synthesis. 2019, 51, 2237–2251.
-
- Snider BB; Lin H Total synthesis of (−)-FR901483. J. Am. Chem. Soc. 1999, 121, 7778–7786.
- Scheffler G; Seike H; Sorensen EJ An enantiospecific synthesis of the potent immunosuppressant FR901483. Angew. Chem. Int. Ed. Engl. 2000, 39, 4593–4596. - PubMed
- Suzuki H; Yamazaki N; Kibayashi C Synthesis of the azatricyclic core of FR901483 by bridgehead vinylation via an anti-Bredt iminium ion. Tetrahedron lett. 2001, 42, 3013–3015.
- Ousmer M; Braun NA; Ciufolini MA Total synthesis of FR901483. Org. Lett. 2001, 3, 765–767. - PubMed
- Maeng JH; Funk RL Total synthesis of the immunosuppressant FR901483 via an amidoacrolein cycloaddition. Org. Lett. 2001, 3, 1125–1128. - PubMed
- Brummond KM; Lu J Tandem cationic aza-Cope rearrangement–Mannich cyclization approach to the core structure of FR901483 via a bridgehead iminium ion. Org. Lett. 2001, 3, 1347–1349. - PubMed
- Ousmer M; Braun NA; Bavoux C; Perrin M; Ciufolini MA Total synthesis of tricyclic azaspirane derivatives of tyrosine: FR901483 and TAN1251C. J. Am. Chem. Soc. 2001, 123, 7534–7538. - PubMed
- Kan T; Fujimoto T; Ieda S; Asoh Y; Kitaoka H; Fukuyama T Stereocontrolled total synthesis of potent immunosuppressant FR901483. Org. Lett. 2004, 6, 2729–2731. - PubMed
- Brummond KM; Hong SP A formal total synthesis of (−)-FR901483, using a tandem cationic aza-Cope rearrangement/Mannich cyclization approach. J. Org. Chem. 2005, 70, 907–916. - PubMed
- Kropf JE; Meigh IC; Bebbington MW; Weinreb SM Studies on a total synthesis of the microbial immunosuppresive agent FR901483. J. Org. Chem. 2006, 71, 2046–2055. - PMC - PubMed
- Diaba F; Ricou E; Solé D; Teixidó E; Valls N; Bonjoch J Studies in the FR901483 tricyclic skeleton synthesis and a new approach to the perhydropyrrolo[2,1-i]indole ring system. Arkivoc. 2007, iv, 320–330.
- Seike H; Sorensen EJ; A synthesis of the tricyclic core structure of FR901483 featuring an Ugi four-component coupling and a remarkably selective elimination reaction. Synlett. 2008. 5, 695–701. - PMC - PubMed
- Carson C; Kerr MA Total synthesis of FR901483. Org. Lett. 2009, 8, 777–779. - PubMed
- Ma AJ; Tu YQ; Peng JB; Dou QY; Hou SH; Zhang FM; Wang SH Total synthesis of (−)-FR901483. Org. Lett. 2012, 12, 3604–3607. - PubMed
- Huo HH; Zhang HK; Xia XE; Huang PQ A formal enantioselective total synthesis of (−)-FR901483. Org. Lett. 2012, 12, 4834–4837. - PubMed
- Perreault S; Rovis T Enantioselective synthesis of the tricyclic core of FR901483 featuring a Rhodium-catalyzed [2+2+2] cycloaddition. Synthesis. 2013, 45, 0719–0728. - PMC - PubMed
- Huo HH; Xia XE; Zhang HK; Huang PQ Enantioselective total syntheses of (−)-FR901483 and (+)-8-epi-FR901483. J. Org. Chem. 2013, 78, 455–465. - PubMed
- Reich D; Trowbridge A; Gaunt MJ Rapid Syntheses of (−)-FR901483 and (+)-TAN1251C Enabled by Complexity-Generating Photocatalytic Olefin Hydroaminoalkylation. Angew. Chem. Int. Ed. Engl. 2020, 59, 2256–2261. - PubMed
-
- Fricke J; Blei F; Hoffmeister D Enzymatic Synthesis of Psilocybin. Angew. Chem. Int. Ed. Engl. 2017, 56, 12352–12355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

