Active-Site Controlled, Jahn-Teller Enabled Regioselectivity in Reductive S-C Bond Cleavage of S-Adenosylmethionine in Radical SAM Enzymes
- PMID: 33372786
- PMCID: PMC7934139
- DOI: 10.1021/jacs.0c10925
Active-Site Controlled, Jahn-Teller Enabled Regioselectivity in Reductive S-C Bond Cleavage of S-Adenosylmethionine in Radical SAM Enzymes
Abstract
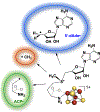
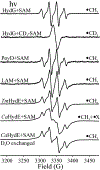
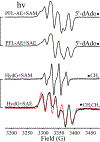
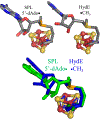
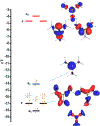
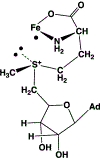
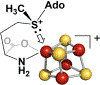
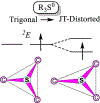
Catalysis by canonical radical S-adenosyl-l-methionine (SAM) enzymes involves electron transfer (ET) from [4Fe-4S]+ to SAM, generating an R3S0 radical that undergoes regioselective homolytic reductive cleavage of the S-C5' bond to generate the 5'-dAdo· radical. However, cryogenic photoinduced S-C bond cleavage has regioselectively yielded either 5'-dAdo· or ·CH3, and indeed, each of the three SAM S-C bonds can be regioselectively cleaved in an RS enzyme. This diversity highlights a longstanding central question: what controls regioselective homolytic S-C bond cleavage upon SAM reduction? We here provide an unexpected answer, founded on our observation that photoinduced S-C bond cleavage in multiple canonical RS enzymes reveals two enzyme classes: in one, photolysis forms 5'-dAdo·, and in another it forms ·CH3. The identity of the cleaved S-C bond correlates with SAM ribose conformation but not with positioning and orientation of the sulfonium center relative to the [4Fe-4S] cluster. We have recognized the reduced-SAM R3S0 radical is a (2E) state with its antibonding unpaired electron in an orbital doublet, which renders R3S0 Jahn-Teller (JT)-active and therefore subject to vibronically induced distortion. Active-site forces induce a JT distortion that localizes the odd electron in a single priority S-C antibond, which undergoes regioselective cleavage. In photolytic cleavage those forces act through control of the ribose conformation and are transmitted to the sulfur via the S-C5' bond, but during catalysis thermally induced conformational changes that enable ET from a cluster iron generate dominant additional forces that specifically select S-C5' for cleavage. This motion also can explain how 5'-dAdo· subsequently forms the organometallic intermediate Ω.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

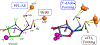
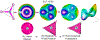

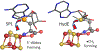
References
-
- Frey PA; Hegeman AD; Ruzicka FJ The Radical SAM Superfamily. Crit. Rev. Biochem. Mol. Biol 2008, 43 (1), 63–88. - PubMed
-
- Nicolet Y Structure–function relationships of radical SAM enzymes. Nature Catalysis 2020, 3 (4), 337–350.
-
- Landgraf BJ; McCarthy EL; Booker SJ Radical S-Adenosylmethionine Enzymes in Human Health and Disease. Annu. Rev. Biochem 2016, 85 (1), 485–514. - PubMed
-
- Holliday GL; Akiva E; Meng EC; Brown SD; Calhoun S; Pieper U; Sali A; Booker SJ; Babbitt PC Chapter One—Atlas of the Radical SAM Superfamily: Divergent Evolution of Function Using a “Plug and Play” Domain. In Methods in Enzymology; Bandarian V, Ed.; Academic Press: 2018; Vol. 606, pp 1–71. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

