Circadian immune circuits
- PMID: 33372990
- PMCID: PMC7774593
- DOI: 10.1084/jem.20200798
Circadian immune circuits
Abstract
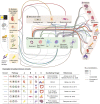
Immune responses are gated to protect the host against specific antigens and microbes, a task that is achieved through antigen- and pattern-specific receptors. Less appreciated is that in order to optimize responses and to avoid collateral damage to the host, immune responses must be additionally gated in intensity and time. An evolutionary solution to this challenge is provided by the circadian clock, an ancient time-keeping mechanism that anticipates environmental changes and represents a fundamental property of immunity. Immune responses, however, are not exclusive to immune cells and demand the coordinated action of nonhematopoietic cells interspersed within the architecture of tissues. Here, we review the circadian features of innate immunity as they encompass effector immune cells as well as structural cells that orchestrate their responses in space and time. We finally propose models in which the central clock, structural elements, and immune cells establish multidirectional circadian circuits that may shape the efficacy and strength of immune responses and other physiological processes.
© 2020 Palomino-Segura and Hidalgo.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

References
-
- A-Gonzalez, N., Quintana J.A., García-Silva S., Mazariegos M., González de la Aleja A., Nicolás-Ávila J.A., Walter W., Adrover J.M., Crainiciuc G., Kuchroo V.K., et al. 2017. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med. 214:1281–1296. 10.1084/jem.20161375 - DOI - PMC - PubMed
-
- Acharya, N., Madi A., Zhang H., Klapholz M., Escobar G., Dulberg S., Christian E., Ferreira M., Dixon K.O., Fell G., et al. 2020. Endogenous Glucocorticoid Signaling Regulates CD8+ T Cell Differentiation and Development of Dysfunction in the Tumor Microenvironment. Immunity. 53:658–671.e6. 10.1016/j.immuni.2020.08.005 - DOI - PMC - PubMed
-
- Adrover, J.M., Del Fresno C., Crainiciuc G., Cuartero M.I., Casanova-Acebes M., Weiss L.A., Huerga-Encabo H., Silvestre-Roig C., Rossaint J., Cossío I., et al. 2019. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity. 50:390–402.e10. 10.1016/j.immuni.2019.01.002 - DOI - PubMed

