Augmented Reduced-Intensity Regimen Does Not Improve Postallogeneic Transplant Outcomes in Acute Myeloid Leukemia
- PMID: 33373276
- PMCID: PMC8078252
- DOI: 10.1200/JCO.20.02308
Augmented Reduced-Intensity Regimen Does Not Improve Postallogeneic Transplant Outcomes in Acute Myeloid Leukemia
Abstract
Purpose: Reduced-intensity conditioning (RIC) regimens have extended the curative potential of allogeneic stem-cell transplantation to older adults with high-risk acute myeloid leukemia (AML) and myelodysplasia (MDS) but are associated with a high risk of disease relapse. Strategies to reduce recurrence are urgently required. Registry data have demonstrated improved outcomes using a sequential transplant regimen, fludarabine/amsacrine/cytarabine-busulphan (FLAMSA-Bu), but the impact of this intensified conditioning regimen has not been studied in randomized trials.
Patients and methods: Two hundred forty-four patients (median age, 59 years) with high-risk AML (n = 164) or MDS (n = 80) were randomly assigned 1:1 to a fludarabine-based RIC regimen or FLAMSA-Bu. Pretransplant measurable residual disease (MRD) was monitored by flow cytometry (MFC-MRD) and correlated with outcome.
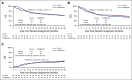
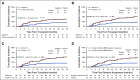
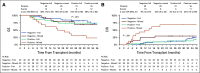
Results: There was no difference in 2-year overall survival (hazard ratio 1.05 [85% CI, 0.80 to 1.38] P = .81) or cumulative incidence of relapse (CIR) (hazard ratio 0.94 [95%CI, 0.60 to 1.46] P = .81) between the control and FLAMSA-Bu arms. Detectable pretransplant MFC-MRD was associated with an increased CIR (2-year CIR 41.0% v 20.0%, P = .01) in the overall trial cohort with a comparable prognostic impact when measured by an unsupervised analysis approach. There was no evidence of interaction between MRD status and conditioning regimen intensity for relapse or survival. Acquisition of full donor T-cell chimerism at 3 months abrogated the adverse impact of pretransplant MRD on CIR and overall survival.
Conclusion: The intensified RIC conditioning regimen, FLAMSA-Bu, did not improve outcomes in adults transplanted for high-risk AML or MDS regardless of pretransplant MRD status. Our data instead support the exploration of interventions with the ability to accelerate acquisition of full donor T-cell chimerism as a tractable strategy to improve outcomes in patients allografted for AML.
Figures

References
-
- Jethava YS Sica S Savani B, et al. : Conditioning regimens for allogeneic hematopoietic stem cell transplants in acute myeloid leukemia. Bone Marrow Transpl 52:1504-1511, 2017 - PubMed
-
- Beelen DW Trenschel R Stelljes M, et al. : Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT. 14/L): A randomised, non-inferiority, phase 3 trial. Lancet Haematol 7:e28-e39, 2020 - PubMed
-
- Schmid C Schleuning M Ledderose G, et al. : Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol 23:5675-5687, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

