Assessing the risk of ketoacidosis due to sodium-glucose cotransporter (SGLT)-2 inhibitors in patients with type 1 diabetes: A meta-analysis and meta-regression
- PMID: 33373368
- PMCID: PMC7771708
- DOI: 10.1371/journal.pmed.1003461
Assessing the risk of ketoacidosis due to sodium-glucose cotransporter (SGLT)-2 inhibitors in patients with type 1 diabetes: A meta-analysis and meta-regression
Abstract
Background: Sodium-glucose cotransporter-2 (SGLT2) inhibitors (SGLT2i) showed benefits in type 1 diabetes mellitus (T1DM), but the risk of diabetic ketoacidosis (DKA) limits their use. Ability to predict DKA risk and therapeutic responses would enable appropriate patient selection for SGLT2i. We conducted a meta-analysis and meta-regression of randomized controlled trials (RCTs) evaluating SGLT2i in T1DM to assess moderators of the relative risk (RR) of DKA, of glycemic (HbA1c, fasting plasma glucose, continuous glucose monitoring parameters, insulin dose, and insulin sensitivity indices) and non-glycemic (body mass index (BMI), systolic BP, renal function, albuminuria, and diabetic eye disorders) efficacy, and of other safety outcomes (including hypoglycemia, infections, major adverse cardiovascular events, and death).
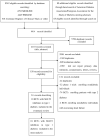
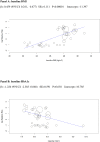
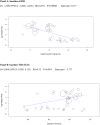
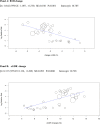
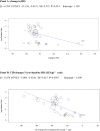
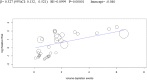
Methods and findings: We searched MEDLINE, Cochrane Library, EMBASE, ClinicalTrials.gov, Cochrane CENTRAL Register of Controlled Trials, and other electronic sources through August 30, 2020, for RCTs comparing SGLT2i with active comparators or placebo in adult patients with T1DM. Reviewers extracted data for relevant outcomes, performed random effects meta-analyses, subgroup analyses, and multivariable meta-regression. The strength of evidence was summarized with the GRADE approach. Among 9,914 records identified, 18 placebo-controlled RCTs (7,396 participants, 50% males, mean age 42 y (range 23 to 55 y), 5 different SGLT2i evaluated), were included. Main outcome measures were effect sizes and moderators of glycemic and non-glycemic efficacy and of safety outcomes. In a multivariable meta-regression model, baseline BMI (β = 0.439 [95% CI: 0.211, 0.666], p < 0.001) and estimated glucose disposal rate (eGDR) (β = -0.766 [-1.276, -0.256], p = 0.001) were associated with the RR of DKA (RR: 2.81; 95% CI:1.97, 4.01; p < 0.001, R2 = 61%). A model including also treatment-related parameters (insulin dose change-to-baseline insulin sensitivity ratio and volume depletion) explained 86% of variance across studies in the risk of DKA (R2 = 86%). The association of DKA with a BMI >27 kg/m2 and with an eGDR <8.3 mg/kg/min was confirmed also in subgroup analyses. Among efficacy outcomes, the novel findings were a reduction in albuminuria (WMD: -9.91, 95% CI: -16.26, -3.55 mg/g, p = 0.002), and in RR of diabetic eye disorders (RR: 0.27[0.11, 0.67], p = 0.005) associated with SGLT2i. A SGLT2i dose-response gradient was consistently observed for main efficacy outcomes, but not for adverse events (AEs). Overall, predictors of DKA and of other AEs differed substantially from those of glycemic and non-glycemic efficacy. A limitation of our analysis was the relatively short (≤52 weeks) duration of included RCTs. The potential relevance for clinical practice needs also to be confirmed by real-world prospective studies.
Conclusions: In T1DM, the risk of DKA and main therapeutic responses to SGLT2i are modified by baseline BMI and insulin resistance, by total insulin dose reduction-to-baseline insulin sensitivity ratio, and by volume depletion, which may enable the targeted use of these drugs in patients with the greatest benefit and the lowest risk of DKA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- US Center for Disease Control and Prevention; http://www.cdc.gov/diabetes/pubs/statsreport14/diabetesinfographic.pdf; accessed November 30th 2018.
-
- Patterson CC, Harjutsa lo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62:408–17. 10.1007/s00125-018-4763-3 - DOI - PubMed
-
- Grempler R, Thomas L, Eckhardt M, Sauer A, Sharp DE, Bakker RA, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. 10.1111/j.1463-1326.2011.01517.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

