Phase I trial of intracerebral convection-enhanced delivery of carboplatin for treatment of recurrent high-grade gliomas
- PMID: 33373402
- PMCID: PMC7771668
- DOI: 10.1371/journal.pone.0244383
Phase I trial of intracerebral convection-enhanced delivery of carboplatin for treatment of recurrent high-grade gliomas
Abstract
Background: Carboplatin is a potent cytoreductive agent for a variety of solid tumors. However, when delivered systemically, clinical efficacy for the treatment of high grade gliomas is poor due to limited penetration across the blood-brain barrier (BBB). Direct intracerebral (IC) convection-enhanced delivery (CED) of carboplatin has been used to bypass the BBB and successfully treat the F98 rat glioma. Based on these studies, we initiated a Phase I clinical trial.
Objective: This Phase I clinical trial was conducted to establish the maximum tolerated dose and define the toxicity profile of carboplatin delivered intracerebrally via convection enhanced delivery (CED) for patients with high grade glial neoplasms.
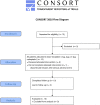
Methods: Cohorts of 3 patients with recurrent WHO grade III or IV gliomas were treated with escalating doses of CED carboplatin (1-4 μg in 54mL over 72 hours) delivered via catheters placed at the time of recurrent tumor resection. The primary outcome measure was determination of the maximum tolerated dose (MTD). Secondary outcome measures included overall survival (OS), progression-free survival (PFS), and radiographic correlation.
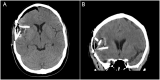
Results: A total of 10 patients have completed treatment with infusion doses of carboplatin of 1μg, 2μg, and 4μg. The total planned volume of infusion was 54mL for each patient. All patients had previously received surgery and chemoradiation. Histology at treatment include GBM (n = 9) and anaplastic oligodendroglioma (n = 1). Median KPS was 90 (range, 70 to 100) at time of treatment. Median PFS and OS were 2.1 and 9.6 months after completion of CED, respectively. A single adverse event possibly related to treatment was noted (generalized seizure).
Conclusions: IC CED of carboplatin as a potential therapy for recurrent malignant glioma is feasible and safe at doses up to 4μg in 54mL over 72 hours. Further studies are needed to determine the maximum tolerated dose and potential efficacy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical