β-Lactams against the Fortress of the Gram-Positive Staphylococcus aureus Bacterium
- PMID: 33373523
- PMCID: PMC8653850
- DOI: 10.1021/acs.chemrev.0c01010
β-Lactams against the Fortress of the Gram-Positive Staphylococcus aureus Bacterium
Abstract
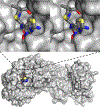
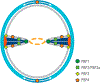
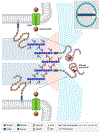
The biological diversity of the unicellular bacteria-whether assessed by shape, food, metabolism, or ecological niche-surely rivals (if not exceeds) that of the multicellular eukaryotes. The relationship between bacteria whose ecological niche is the eukaryote, and the eukaryote, is often symbiosis or stasis. Some bacteria, however, seek advantage in this relationship. One of the most successful-to the disadvantage of the eukaryote-is the small (less than 1 μm diameter) and nearly spherical Staphylococcus aureus bacterium. For decades, successful clinical control of its infection has been accomplished using β-lactam antibiotics such as the penicillins and the cephalosporins. Over these same decades S. aureus has perfected resistance mechanisms against these antibiotics, which are then countered by new generations of β-lactam structure. This review addresses the current breadth of biochemical and microbiological efforts to preserve the future of the β-lactam antibiotics through a better understanding of how S. aureus protects the enzyme targets of the β-lactams, the penicillin-binding proteins. The penicillin-binding proteins are essential enzyme catalysts for the biosynthesis of the cell wall, and understanding how this cell wall is integrated into the protective cell envelope of the bacterium may identify new antibacterials and new adjuvants that preserve the efficacy of the β-lactams.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
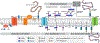








References
-
- David MZ The importance of Staphylococcus aureus genotypes in outcomes and complications of bacteremia. Clin. Infect. Dis 2019, 69, 1878–1880. - PubMed
-
- Rello J; Eshwara VK; Lagunes L; Alves J; Wunderink RG; Conway-Morris A; Rojas JN; Alp E; Zhang Z A global priority list of the TOp TEn resistant Microorganisms (TOTEM) study at intensive care: a prioritization exercise based on multi-criteria decision analysis. Eur. J. Clin. Microbiol. Infect. Dis 2019, 38, 319–323. - PubMed
-
- Talbot GH; Jezek A; Murray BE; Jones RN; Ebright RH; Nau GJ; Rodvold KA; Newland JG; Boucher HW The Infectious Diseases Society of America’s 10 × ‘20 initiative (ten new systemic antibacterial agents FDA-approved by 2020): Is 20 × ‘20 a possibility? Clin. Infect. Dis 2019, 69, 1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

