Adverse effects of hydroxychloroquine and azithromycin on contractility and arrhythmogenicity revealed by human engineered cardiac tissues
- PMID: 33373642
- PMCID: PMC7765761
- DOI: 10.1016/j.yjmcc.2020.12.014
Adverse effects of hydroxychloroquine and azithromycin on contractility and arrhythmogenicity revealed by human engineered cardiac tissues
Abstract
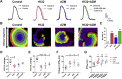
The coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic as declared by World Health Organization (WHO). In the absence of an effective treatment, different drugs with unknown effectiveness, including antimalarial hydroxychloroquine (HCQ), with or without concurrent administration with azithromycin (AZM), have been tested for treating COVID-19 patients with developed pneumonia. However, the efficacy and safety of HCQ and/or AZM have been questioned by recent clinical reports. Direct effects of these drugs on the human heart remain very poorly defined. To better understand the mechanisms of action of HCQ +/- AZM, we employed bioengineered human ventricular cardiac tissue strip (hvCTS) and anisotropic sheet (hvCAS) assays, made with human pluripotent stem cell (hPSC)-derived ventricular cardiomyocytes (hvCMs), which have been designed for measuring cardiac contractility and electrophysiology, respectively. Our hvCTS experiments showed that AZM induced a dose-dependent negative inotropic effect which could be aggravated by HCQ; electrophysiologically, as revealed by the hvCAS platform, AZM prolonged action potentials and induced spiral wave formations. Collectively, our data were consistent with reported clinical risks of HCQ and AZM on QTc prolongation/ventricular arrhythmias and development of heart failure. In conclusion, our study exposed the risks of HCQ/AZM administration while providing mechanistic insights for their toxicity. Our bioengineered human cardiac tissue constructs therefore provide a useful platform for screening cardiac safety and efficacy when developing therapeutics against COVID-19.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
-
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. - PMC - PubMed
-
- Bull-Otterson L., Gray E.B., Budnitz D.S., Strosnider H.M., Schieber L.Z., Courtney J., Garcia M.C., Brooks J.T., Mac Kenzie W.R., Gundlapalli A.V. Hydroxychloroquine and chloroquine prescribing patterns by provider specialty following initial reports of potential benefit for COVID-19 treatment - United States, January-June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1210–1215. - PMC - PubMed
-
- Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020;50:384. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

