Decreased plasma rifapentine concentrations associated with AADAC single nucleotide polymorphism in adults with tuberculosis
- PMID: 33374006
- PMCID: PMC7879139
- DOI: 10.1093/jac/dkaa490
Decreased plasma rifapentine concentrations associated with AADAC single nucleotide polymorphism in adults with tuberculosis
Abstract
Background: Rifapentine exposure is associated with bactericidal activity against Mycobacterium tuberculosis, but high interindividual variation in plasma concentrations is encountered.
Objectives: To investigate a genomic association with interindividual variation of rifapentine exposure, SNPs of six human genes involving rifamycin metabolism (AADAC, CES2), drug transport (SLCO1B1, SLCO1B3) and gene regulation (HNF4A, PXR) were evaluated.
Methods: We characterized these genes in 173 adult participants in treatment trials of the Tuberculosis Trials Consortium. Participants were stratified by self-identified race (black or non-black), and rifapentine AUC from 0 to 24 h (AUC0-24) was adjusted by analysis of covariance for SNPs, rifapentine dose, sex, food and HIV coinfection. This study was registered at ClinicalTrials.gov under identifier NCT01043575.
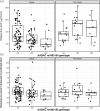
Results: The effect on rifapentine least squares mean AUC0-24 in black participants overall decreased by -10.2% for AADAC rs1803155 G versus A allele (Wald test: P = 0.03; false discovery rate, 0.10). Black participants with one G allele in AADAC rs1803155 were three times as likely to have below target bactericidal rifapentine exposure than black participants with the A allele (OR, 2.97; 95% CI: 1.16, 7.58). With two G alleles, the OR was greater. In non-black participants, AADAC rs1803155 SNP was not associated with rifapentine exposure. In both black and non-black participants, other evaluated genes were not associated with rifapentine exposure (P > 0.05; false discovery rate > 0.10).
Conclusions: Rifapentine exposure in black participants varied with AADAC rs1803155 genotype and the G allele was more likely to be associated with below bactericidal target rifapentine exposure. Further pharmacogenomic study is needed to characterize the association of the AADAC rs1803155 with inadequate rifapentine exposure in different patient groups.
Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy 2020. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
Similar articles
-
Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB.Cochrane Database Syst Rev. 2013 Jul 5;2013(7):CD007545. doi: 10.1002/14651858.CD007545.pub2. Cochrane Database Syst Rev. 2013. PMID: 23828580 Free PMC article.
-
A Population Pharmacokinetic Analysis Shows that Arylacetamide Deacetylase (AADAC) Gene Polymorphism and HIV Infection Affect the Exposure of Rifapentine.Antimicrob Agents Chemother. 2019 Mar 27;63(4):e01964-18. doi: 10.1128/AAC.01964-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30670438 Free PMC article. Clinical Trial.
-
Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children.Cochrane Database Syst Rev. 2022 Sep 6;9(9):CD013359. doi: 10.1002/14651858.CD013359.pub3. Cochrane Database Syst Rev. 2022. PMID: 36065889 Free PMC article.
-
Safety, bactericidal activity, and pharmacokinetics of the antituberculosis drug candidate BTZ-043 in South Africa (PanACEA-BTZ-043-02): an open-label, dose-expansion, randomised, controlled, phase 1b/2a trial.Lancet Microbe. 2025 Feb;6(2):100952. doi: 10.1016/j.lanmic.2024.07.015. Epub 2025 Jan 7. Lancet Microbe. 2025. PMID: 39793592 Clinical Trial.
-
Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance.Cochrane Database Syst Rev. 2018 Aug 27;8(8):CD012768. doi: 10.1002/14651858.CD012768.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 Jan 15;1:CD012768. doi: 10.1002/14651858.CD012768.pub3. PMID: 30148542 Free PMC article. Updated.
Cited by
-
Pharmacogenetics of plasma dolutegravir exposure during 1-month rifapentine/isoniazid treatment of latent tuberculosis.Pharmacogenet Genomics. 2025 Jun 1;35(4):140-144. doi: 10.1097/FPC.0000000000000562. Epub 2025 Feb 12. Pharmacogenet Genomics. 2025. PMID: 39960813 Free PMC article.
-
Exploring Variability in Rifampicin Plasma Exposure and Development of Anti-Tuberculosis Drug-Induced Liver Injury among Patients with Pulmonary Tuberculosis from the Pharmacogenetic Perspective.Pharmaceutics. 2024 Mar 12;16(3):388. doi: 10.3390/pharmaceutics16030388. Pharmaceutics. 2024. PMID: 38543282 Free PMC article.
-
Pharmacogenetics as part of recommended precision medicine for tuberculosis treatment in African populations: Could it be a reality?Clin Transl Sci. 2023 Jul;16(7):1101-1112. doi: 10.1111/cts.13520. Epub 2023 Jun 8. Clin Transl Sci. 2023. PMID: 37291686 Free PMC article. Review.
-
Progress of arylacetamide deacetylase research in metabolic diseases.Front Oncol. 2025 May 1;15:1564419. doi: 10.3389/fonc.2025.1564419. eCollection 2025. Front Oncol. 2025. PMID: 40376582 Free PMC article. Review.
-
Effect of Genetic Variations in Drug-Metabolizing Enzymes and Drug Transporters on the Pharmacokinetics of Rifamycins: A Systematic Review.Pharmgenomics Pers Med. 2022 Jun 4;15:561-571. doi: 10.2147/PGPM.S363058. eCollection 2022. Pharmgenomics Pers Med. 2022. PMID: 35693129 Free PMC article. Review.
References
-
- Dorman SE, Goldberg S, Stout JE. et al. Substitution of rifapentine for rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the Tuberculosis Trials Consortium. J Infect Dis 2012; 206: 1030–40. - PubMed
-
- Dorman SE, Nahid P, Kurbatova EV et . Tuberculosis Consortium. High dose rifapentine with or without moxifloxacin for shortening treatment of pulmonary tuberculosis: Study protocol for TBTC Study 31/ACTG A5349 phase 3 clinical trial. Contemp Clin Trials 2020; doi:10.1016/j.cct.2020.105938. - PMC - PubMed
-
- Nakajima A, Fukami T, Kobayashi Y. et al. Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: rifampicin, rifabutin, and rifapentine. Biochem Pharmacol 2011; 82: 1747–56. - PubMed

