Manipulating the Metabolism to Improve the Efficacy of CAR T-Cell Immunotherapy
- PMID: 33374128
- PMCID: PMC7824126
- DOI: 10.3390/cells10010014
Manipulating the Metabolism to Improve the Efficacy of CAR T-Cell Immunotherapy
Abstract
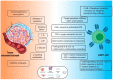
The adoptive transfer of the chimeric antigen receptor (CAR) expressing T-cells has produced unprecedented successful results in the treatment of B-cell malignancies. However, the use of this technology in other malignancies remains less effective. In the setting of solid neoplasms, CAR T-cell metabolic fitness needs to be optimal to reach the tumor and execute their cytolytic function in an environment often hostile. It is now well established that both tumor and T cell metabolisms play critical roles in controlling the immune response by conditioning the tumor microenvironment and the fate and activity of the T cells. In this review, after a brief description of the tumoral and T cell metabolic reprogramming, we summarize the latest advances and new strategies that have been developed to improve the metabolic fitness and efficacy of CAR T-cell products.
Keywords: Chimeric Antigen Receptor T cells; cancer; combined therapy; immunotherapy; metabolic reprogramming.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
Manipulating the tumor microenvironment by adoptive cell transfer of CAR T-cells.Mamm Genome. 2018 Dec;29(11-12):739-756. doi: 10.1007/s00335-018-9756-5. Epub 2018 Jul 9. Mamm Genome. 2018. PMID: 29987406 Review.
-
Chimeric antigen receptor T-cell therapy for cancer: a basic research-oriented perspective.Immunotherapy. 2018 Mar;10(3):221-234. doi: 10.2217/imt-2017-0133. Immunotherapy. 2018. PMID: 29370727
-
Engineering CAR-T Cells for Next-Generation Cancer Therapy.Cancer Cell. 2020 Oct 12;38(4):473-488. doi: 10.1016/j.ccell.2020.07.005. Epub 2020 Jul 30. Cancer Cell. 2020. PMID: 32735779 Review.
-
Advances in CAR-T Cell Genetic Engineering Strategies to Overcome Hurdles in Solid Tumors Treatment.Front Immunol. 2022 Feb 8;13:830292. doi: 10.3389/fimmu.2022.830292. eCollection 2022. Front Immunol. 2022. PMID: 35211124 Free PMC article. Review.
-
Prolonged Persistence of Chimeric Antigen Receptor (CAR) T Cell in Adoptive Cancer Immunotherapy: Challenges and Ways Forward.Front Immunol. 2020 Apr 22;11:702. doi: 10.3389/fimmu.2020.00702. eCollection 2020. Front Immunol. 2020. PMID: 32391013 Free PMC article. Review.
Cited by
-
BOXR1030, an anti-GPC3 CAR with exogenous GOT2 expression, shows enhanced T cell metabolism and improved anti-cell line derived tumor xenograft activity.PLoS One. 2022 May 4;17(5):e0266980. doi: 10.1371/journal.pone.0266980. eCollection 2022. PLoS One. 2022. PMID: 35507536 Free PMC article.
-
Chimeric Antigen Receptor T-Cell Therapy in Paediatric B-Cell Precursor Acute Lymphoblastic Leukaemia: Curative Treatment Option or Bridge to Transplant?Front Pediatr. 2022 Jan 25;9:784024. doi: 10.3389/fped.2021.784024. eCollection 2021. Front Pediatr. 2022. PMID: 35145941 Free PMC article. Review.
-
Targeting memory T cell metabolism to improve immunity.J Clin Invest. 2022 Jan 4;132(1):e148546. doi: 10.1172/JCI148546. J Clin Invest. 2022. PMID: 34981777 Free PMC article. Review.
-
Site-specific transgene integration in chimeric antigen receptor (CAR) T cell therapies.Biomark Res. 2023 Jul 4;11(1):67. doi: 10.1186/s40364-023-00509-1. Biomark Res. 2023. PMID: 37403182 Free PMC article. Review.
-
Humanized Chimeric Antigen Receptor (CAR) T cells.J Cancer Immunol (Wilmington). 2021;3(4):183-187. J Cancer Immunol (Wilmington). 2021. PMID: 35128536 Free PMC article. No abstract available.
References
-
- Sadelain M., Brentjens R., Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. - DOI - PMC - PubMed
-
- Bouchkouj N., Kasamon Y.L., de Claro R.A., George B., Lin X., Lee S., Blumenthal G.M., Bryan W., McKee A.E., Pazdur R. FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Large B-cell Lymphoma. Clin. Cancer Res. 2019;25:1702–1708. doi: 10.1158/1078-0432.CCR-18-2743. - DOI - PubMed
-
- O’Leary M.C., Lu X., Huang Y., Lin X., Mahmood I., Przepiorka D., Gavin D., Lee S., Liu K., George B., et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2019;25:1142–1146. doi: 10.1158/1078-0432.CCR-18-2035. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

