Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights into the Disease Pathophysiology, Diagnosis and Management
- PMID: 33374173
- PMCID: PMC7795410
- DOI: 10.3390/ijms22010100
Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights into the Disease Pathophysiology, Diagnosis and Management
Abstract
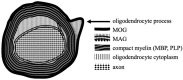
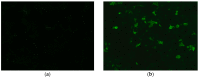
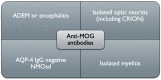
Myelin oligodendrocyte glycoprotein (MOG)-associated disease (MOGAD) is a rare, antibody-mediated inflammatory demyelinating disorder of the central nervous system (CNS) with various phenotypes starting from optic neuritis, via transverse myelitis to acute demyelinating encephalomyelitis (ADEM) and cortical encephalitis. Even though sometimes the clinical picture of this condition is similar to the presentation of neuromyelitis optica spectrum disorder (NMOSD), most experts consider MOGAD as a distinct entity with different immune system pathology. MOG is a molecule detected on the outer membrane of myelin sheaths and expressed primarily within the brain, spinal cord and also the optic nerves. Its function is not fully understood but this glycoprotein may act as a cell surface receptor or cell adhesion molecule. The specific outmost location of myelin makes it a potential target for autoimmune antibodies and cell-mediated responses in demyelinating processes. Optic neuritis seems to be the most frequent presenting phenotype in adults and ADEM in children. In adults, the disease course is multiphasic and subsequent relapses increase disability. In children ADEM usually presents as a one-time incident. Luckily, acute immunotherapy is very effective and severe disability (ambulatory and visual) is less frequent than in NMOSD. A critical element of reliable diagnosis is detection of pathogenic serum antibodies MOG with accurate, specific and sensitive methods, preferably with optimized cell-based assay (CBA). MRI imaging can also help in differentiating MOGAD from other neuro-inflammatory disorders. Reports on randomised control trials are limited, but observational open-label experience suggests a role for high-dose steroids and plasma exchange in the treatment of acute attacks, and for immunosuppressive therapies, such as steroids, oral immunosuppressants and rituximab as maintenance treatment. In this review, we present up-to-date clinical, immunological, radiographic, histopathological data concerning MOGAD and summarize the practical aspects of diagnosing and managing patients with this disease.
Keywords: NMO spectrum disorder; myelin oligodendrocyte glycoprotein (MOG); myelin oligodendrocyte glycoprotein associated disease (MOGAD); neuroimmunology; neuromyelitis optica (NMO).
Conflict of interest statement
All authors declare no conflict of interest.
Figures

References
-
- Schumacher G.A., Beebe G., Kibler R.F., Kurland L.T., Kurtzke J.F., McDowell F., Nagler B., Sibley W.A., Tourtellotte W.W., Willmon T.L. Problems of experimental trials of therapy in multiple sclerosis: Report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann. N. Y. Acad. Sci. 1965;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. - DOI - PubMed
-
- Wingerchuk D.M., Banwell B., Bennett J.L., Cabre P., Carroll W., Chitnis T., De Seze J., Fujihara K., Greenberg B., Jacob A., et al. International consensus diagnostic criteria for neuromyelitis opstica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

