Evaluation of the Efficacy of a Cholera-Toxin-Based Staphylococcus aureus Vaccine against Bovine Intramammary Challenge
- PMID: 33374191
- PMCID: PMC7824273
- DOI: 10.3390/vaccines9010006
Evaluation of the Efficacy of a Cholera-Toxin-Based Staphylococcus aureus Vaccine against Bovine Intramammary Challenge
Abstract
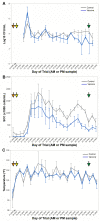
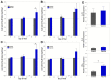
Staphylococcus aureus (S. aureus) is a primary agent of bovine mastitis and a source of significant economic loss for the dairy industry. We previously reported antigen-specific immune induction in the milk and serum of dairy cows following vaccination with a cholera toxin A2 and B subunit (CTA2/B) based vaccine containing the iron-regulated surface determinant A (IsdA) and clumping factor A (ClfA) antigens of S. aureus (IsdA + ClfA-CTA2/B). The goal of the current study was to assess the efficacy of this vaccine to protect against S. aureus infection after intramammary challenge. Six mid-lactation heifers were randomized to vaccinated and control groups. On days 1 and 14 animals were inoculated intranasally with vaccine or vehicle control, and on day 20 animals were challenged with S. aureus. Clinical outcome, milk quality, bacterial shedding, and somatic cell count (SCC) were followed for ten days post-challenge. Vaccinated animals did not show signs of clinical S. aureus mastitis and had lower SCCs compared to control animals during the challenge period. Reductions in bacterial shedding were observed but were not significant between groups. Antibody analysis of milk and serum indicated that, upon challenge, vaccinated animals produced enhanced IsdA- and ClfA-CTA2/B specific immunoglobulin G (IgG) responses, while responses to CTA2/B alone were not different between groups. Responses after challenge were largely IgG1 against the IsdA antigen and mixed IgG1/IgG2 against the ClfA antigen. In addition, there was a significant increase in interferon gamma (IFN-γ) expression from blood cells in vaccinated animals on day 20. While preliminary, these findings support evidence of the induction of active immunity by IsdA + ClfA-CTA2/B, and further assessment of this vaccine is warranted.
Keywords: Staphylococcus aureus; bovine; mastitis; vaccine.
Conflict of interest statement
J.K.T. holds an unlicensed patent for the use of cholera toxin chimera as a staphylococcal vaccine (Tinker, U.S. Pat. No. 8,834,898).
Figures



References
-
- APHIS U. Dairy 2014. Milk Quality, Milking Procedures, and Mastitis on U.S. Dairies. Volume 2016 United States Department of Agriculture; Washington, DC, USA: 2014.
-
- Sacco S.C., Velazquez N.S., Renna M.S., Beccaria C., Baravalle C., Pereyra E.A.L., Monecke S., Calvinho L.F., Dallard B.E. Capacity of two Staphylococcus aureus strains with different adaptation genotypes to persist and induce damage in bovine mammary epithelial cells and to activate macrophages. Microb. Pathog. 2020;142:104017. doi: 10.1016/j.micpath.2020.104017. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

